Table 2.
Extension of the Anti-Selective Reductive Coupling to a Variety of Aryl Alkynes and α-Oxyaldehydesa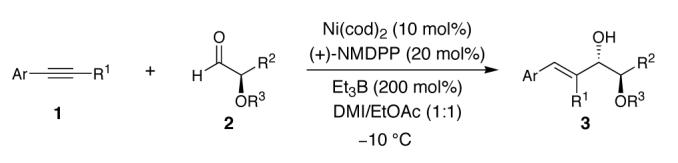
| entry | Ar | R1 | R2 | R3 | d.r.b | yield (%) (d.r.)c |
|---|---|---|---|---|---|---|
| 1 | Ph | Me | Cy | MOM | 88:12 | 87(90:10) |
| 2 | 1-Naphthyl | Me | Cy | MOM | 83:17 | 93(83:17) |
| 3 | Ph | Et | Cy | MOM | >95:5 | 54(>95:5) |
| 4 | Ph | 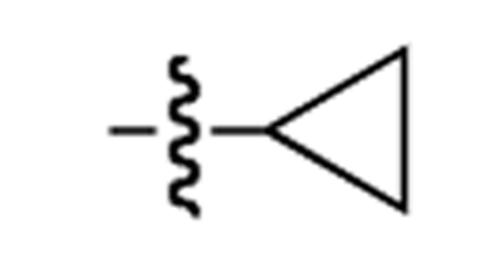 |
Cy | MOM | >95:5 | 62(>95:5) |
| 5 | Ph | CH2NHBoc | Cy | MOM | 90:10 | 60(95:5) |
| 6 | Ph | Et | Cy | PMB | >95:5 | 74(>95:5) |
| 7 | Ph | Et | Ph | MOM | 68:32 | 39(>95:5)d |
| 8 | Ph | Et | n-hexyl | MOM | 75:25 | 86(80:20) |
All reactions were conducted with 100 mol% of alkyne and 150 mol% of aldehyde. All reactions proceeded with >95:5 regioselectivity.
Ratio of anti:syn determined by 1H NMR analysis ofcrude reaction mixtures.
Yield and diastereoselectivity of the isolated reaction product.
The syn product was also isolated in 18% yield (>95:5 d.r.).
