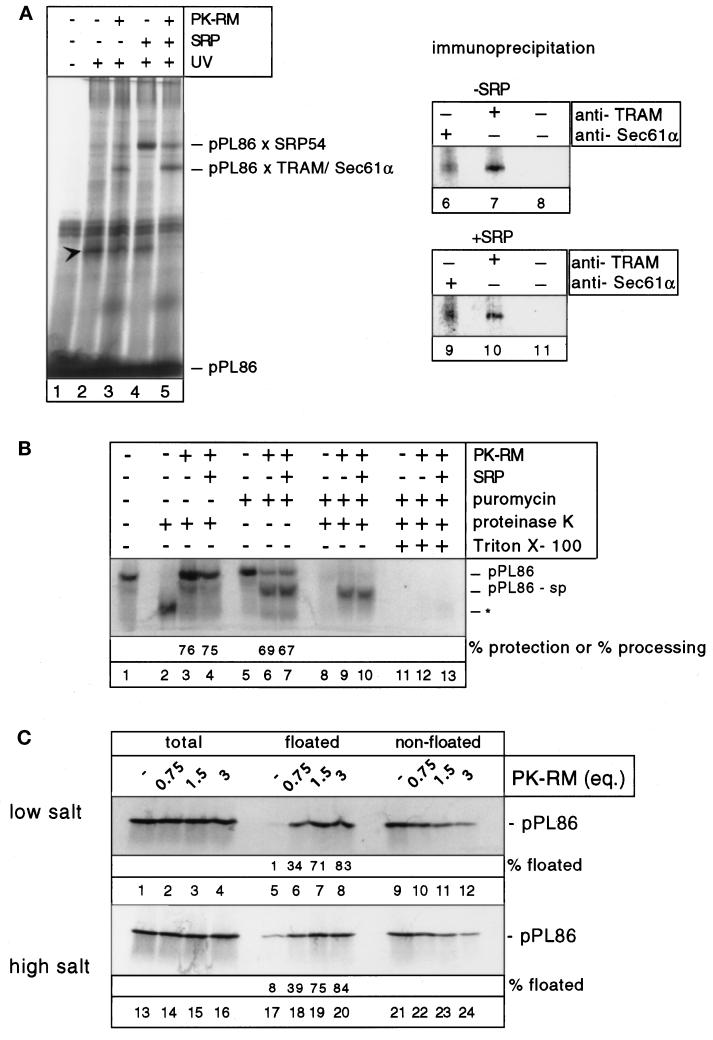
Figure 1.
SRP-independent membrane binding of ribosome/nascent chain complexes. (A) Photocrosslinking of pPL86 to SRP and membrane proteins. pPL86 was synthesized in the wheat germ system in the presence of modified lysyl-tRNA. Dog pancreatic SRP and PK-RM were added as indicated. The samples were UV-irradiated, separated by SDS-PAGE, and analyzed with a PhosphoImager. pPL86 × SRP54 stands for the cross-linked product containing SRP54. To identify the cross-links to membrane proteins (pPL86 × TRAM/Sec61α), immunoprecipitations with antibodies to Sec61α or TRAM, as well as control precipitations without antibodies were carried out (lanes 6–11). Arrowhead denotes a cross-linked product containing an unknown cytosolic protein. (B) Membrane targeting and translocation of pPL86. pPL86 synthesized in the wheat germ system was incubated with PK-RM and SRP as indicated. Some samples (lanes 2–4) were treated with proteinase K, the others (lanes 5–13) with puromycin. To test for translocation after puromycin treatment, the samples were incubated with proteinase K in the absence or presence of Triton X-100. pPL86-sp indicates the nascent chain fragment after signal peptide cleavage. Asterisk indicates the 30-amino acid fragment protected from proteolysis by the ribosome. (C) Determination of membrane targeting by flotation. pPL86 was incubated with different amounts of PK-RM in the absence of SRP. The samples were then layered under a sucrose gradient under low- (150 mM) or high- (500 mM) salt conditions and subjected to centrifugation. The floated and nonfloated fractions were analyzed by SDS-PAGE.