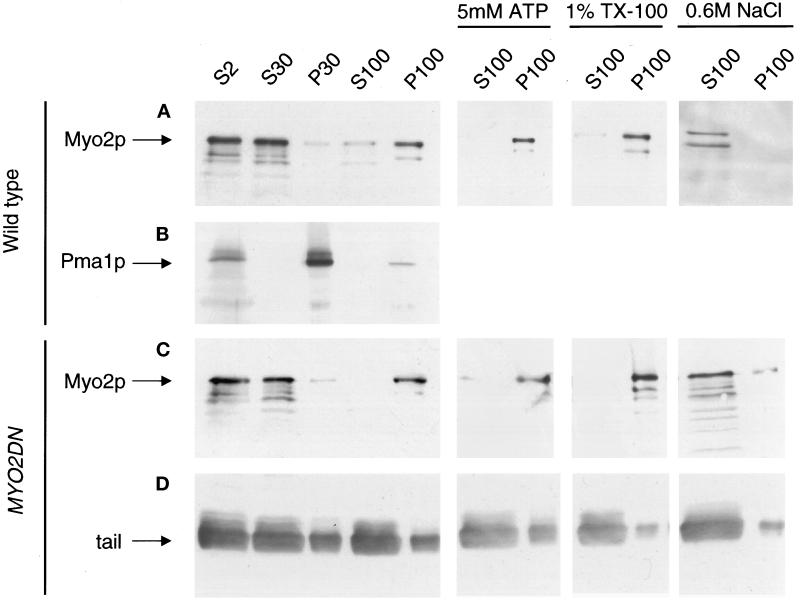
Figure 8.
Subcellular fractionation of wild-type and MYO2DN cells. Wild-type (A and B) and MYO2DN (C and D) cells were grown in galactose for 3 h. Cells were harvested, and the resulting lysate was spun at 2,000 × g to generate supernatant 2 (S2). S2 was spun at 30,000 × g, resulting in S30 and pellet 30 (P30). S30 was spun at 100,000 × g to generate S100 and P100. To investigate the solubility of Myo2p, 5 mM Mg-ATP, 1% Triton X-100, or 0.6 M NaCl was added to S30, which was then spun at 100,000 × g, yielding S100 and P100. Gel samples were prepared using volumetric stoichiometry, and equal volumes of each sample were loaded per lane, separated by SDS-PAGE, and transferred to PVDF. The blots were then probed with anti-Myo2p head antibody (A and C), anti-Pma1p (B), or anti-HA (D). The majority of the Myo2p was found in the 100,000 × g pellet. The Myo2p in this pellet was not released by tail overexpression, Mg–ATP, or Triton X-100, but was solubilized by 0.6 M NaCl.