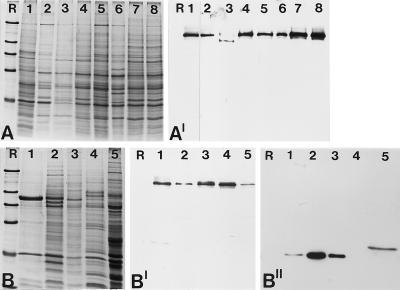
Figure 3.
Identification of the 146-kDa protein in different cell culture lines and nuclear fractions from Xenopus oocytes. (A) Coomassie blue-stained total cellular proteins. Cell lines shown are: X. laevis kidney epithelial cells, line A6 (lane 1); chicken embryonic fibroblasts line CEF (lane 2); rat kangaroo cells, PtK2 (lane 3); embryonal mouse cells of line 3T3-L1 (lane 4); rat vascular smooth muscle-derived cells of line RV (lane 5); bovine kidney epithelial cell line MDBK (lane 6); human primary liver carcinoma cells of line PLC (lane 7); and human cervical adenocarcinoma cells of line HeLa (lane 8). (A′) Corresponding autoradiogram showing immunochemiluminescence detection of the antigenic polypeptide using antibody B2.4–1. A shorter exposure of lane 1 is presented because of the very strong reaction of the antibodies generated against the Xenopus protein. The comparable weak reaction in lane 3 might be due to partial degradation of the protein. (B) Coomassie blue staining of various nuclear fractions of Xenopus oocytes separated by SDS-PAGE. Total mass-isolated nuclei (lane 1); proteins of the LSP, HSP, and HSS fractions of fractionated oocyte nuclei (lanes 2–4); and Xenopus egg extract (lane 5). (B′) Corresponding immunoblot probed with antibody B2.4–1, which specifically reacts with the 146-kDa protein present in all fractions analyzed. (B") Probing of a parallel immunoblot with mAB No-185 directed against the well characterized nucleolar protein NO38 to ascertain the fractionation procedure. Reference proteins (R) are the same as in Figure 2.