Abstract
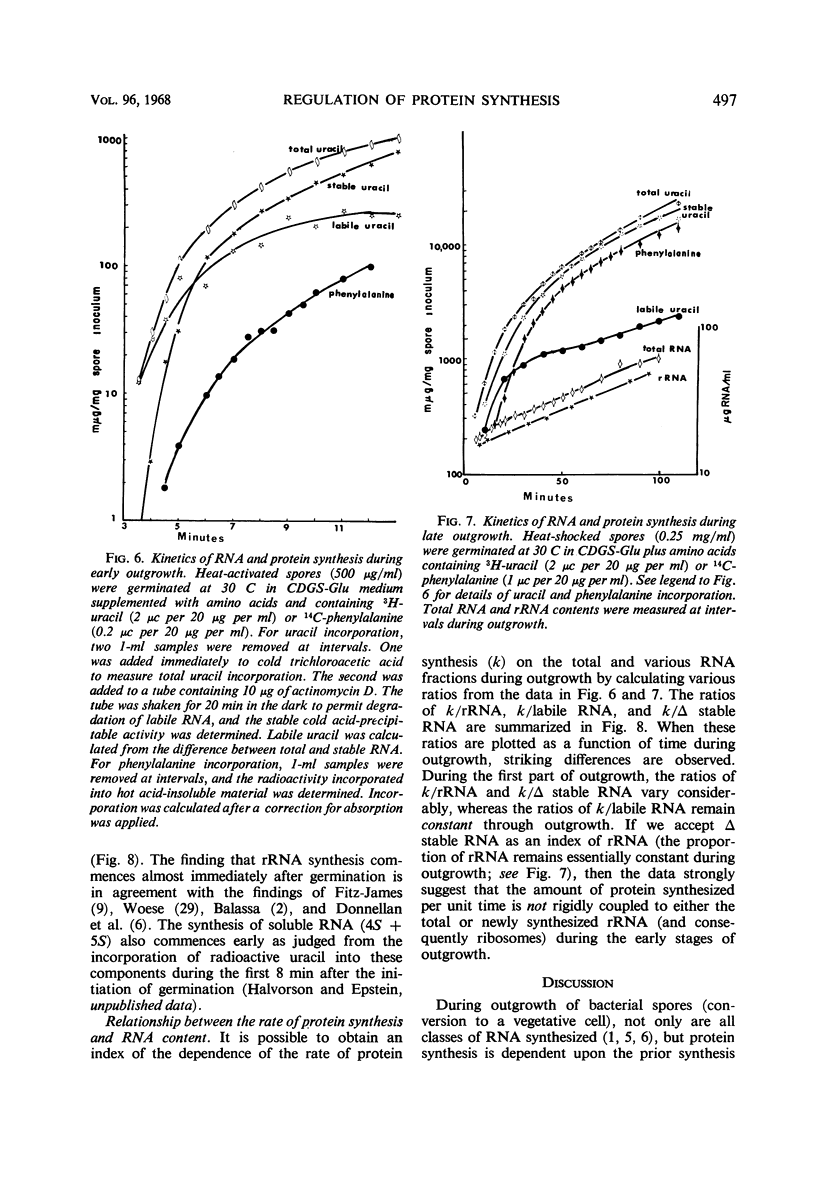
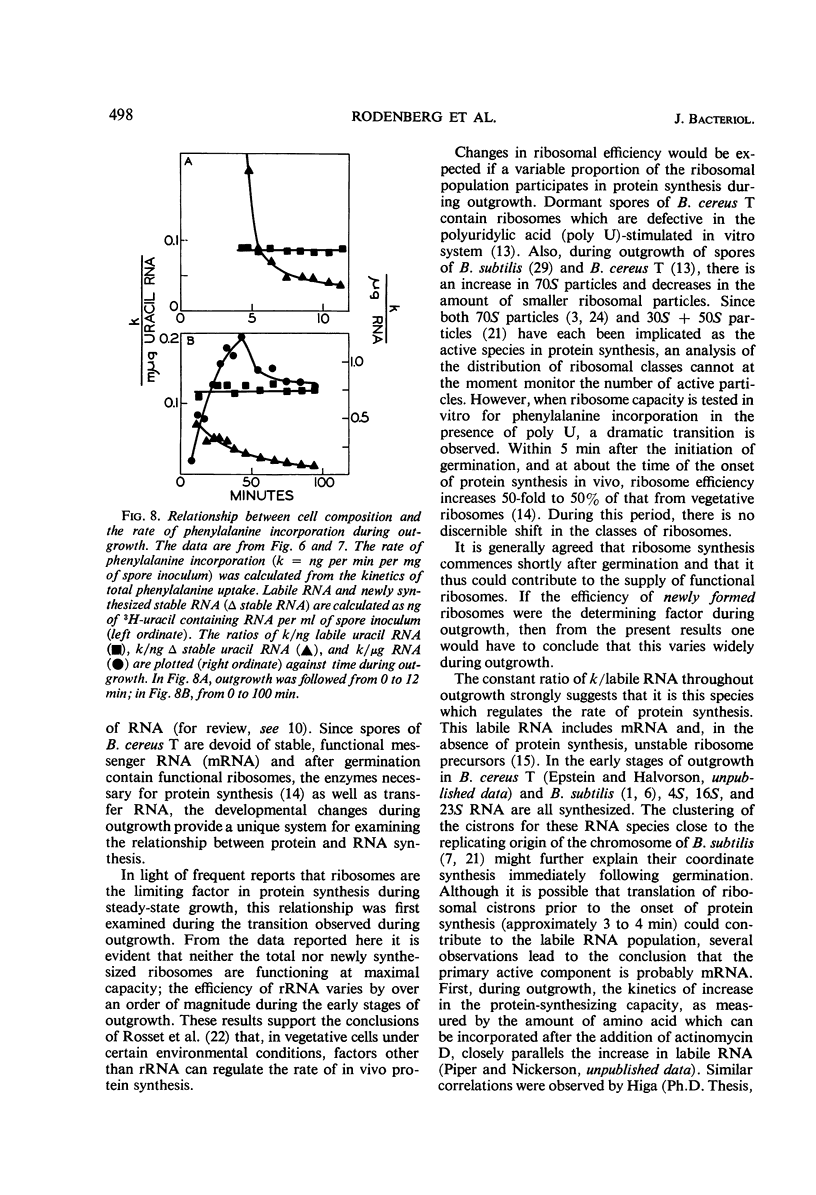
The rate of protein and ribonucleic acid (RNA) synthesis was examined during the outgrowth of spores of Bacillus cereus T in a chemically defined medium. RNA synthesis started 2.5 min after the initiation of germination, and protein synthesis after 4 min. Addition of a complete amino acid supplement and uracil supported high rates of RNA and protein synthesis throughout outgrowth. To determine the relationship between the rate of protein (k) and RNA synthesis, the kinetics of formation of various classes of RNA were followed during outgrowth. Ribosomal RNA (rRNA) comprised a relatively constant fraction of the total RNA throughout outgrowth (71 to 78%). The classes of RNA synthesized during this period were determined by germinating spores in radioactive uracil and then at intervals following their stability to actinomycin D. Initially, labile RNA comprised the largest fraction of newly formed RNA (ΔRNA), and this proportion decreased during outgrowth. The ratio of k/rRNA or k/Δ stable RNA varied considerably during outgrowth, whereas the ratio of k/labile RNA remained constant. The data suggest that the rate of protein synthesis is not rigidly coupled to either total or newly synthesized rRNA (ribosomes) during the early stages of outgrowth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. L., Sueoka N. Phase transitions in ribonucleic acid synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1968 Jan;59(1):153–160. doi: 10.1073/pnas.59.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALASSA G. [Ribonucleic acid from Bacillus subtilis spores]. Biochim Biophys Acta. 1963 Jul 30;72:497–500. doi: 10.1016/0006-3002(63)90272-5. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Marcker K. A. Polypeptidyl-sigma-ribonucleic acid and amino-acyl-sigma-ribonucleic acid binding sites on ribosomes. Nature. 1966 Jul 23;211(5047):380–384. doi: 10.1038/211380a0. [DOI] [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H., HALVORSON H. O. Studies on spore germination: its independence from alanine racemase activity. J Bacteriol. 1954 Oct;68(4):393–399. doi: 10.1128/jb.68.4.393-399.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R. H., IGARASHI R. T. GENETIC TRANSCRIPTION DURING MORPHOGENESIS. Proc Natl Acad Sci U S A. 1964 Sep;52:755–762. doi: 10.1073/pnas.52.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Marmur J. Gene conservation in Bacillus species. II. The location of genes concerned with the synthesis of ribosomal components and soluble RNA. Proc Natl Acad Sci U S A. 1965 Sep;54(3):724–730. doi: 10.1073/pnas.54.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKER R. E., SCHAECHTER M. RIBOSOME CONTENT AND THE RATE OF GROWTH OF SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1963 Oct 15;76:275–279. [PubMed] [Google Scholar]
- FITZ-JAMES P. C. The phosphorus fractions of Bacillus cereus and Bacillus megaterium. II. A correlation of the chemical with the cytological changes occurring during spore germination. Can J Microbiol. 1955 Aug;1(7):525–548. doi: 10.1139/m55-066. [DOI] [PubMed] [Google Scholar]
- Halvorson H. O., Vary J. C., Steinberg W. Developmental changes during the formation and breaking of the dormant state in bacteria. Annu Rev Microbiol. 1966;20:169–188. doi: 10.1146/annurev.mi.20.100166.001125. [DOI] [PubMed] [Google Scholar]
- KENNELL D., MAGASANIK B. The relation of ribosome content to the rate of enzyme synthesis in Aerobacter aerogenes. Biochim Biophys Acta. 1962 Jan 22;55:139–151. doi: 10.1016/0006-3002(62)90940-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Halvorson H. O. Evidence for a defective protein synthesizing system in dormant spores of Bacillus cereus. Arch Biochem Biophys. 1968 Mar 11;123(3):622–632. doi: 10.1016/0003-9861(68)90182-3. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateles R. K., Ryu D. Y., Yasuda T. Measurement of unsteady state growth rates of micro-organisms. Nature. 1965 Oct 16;208(5007):263–265. doi: 10.1038/208263a0. [DOI] [PubMed] [Google Scholar]
- NAKATA H. M. ORGANIC NUTRIENTS REQUIRED FOR GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1964 Nov;88:1522–1524. doi: 10.1128/jb.88.5.1522-1524.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V. PHAGE f2 RNA-DIRECTED BINDING OF FORMYLMETHIONYL-TRNA TO RIBOSOMES AND THE ROLE OF 30S RIBOSOMAL SUBUNITS IN INITIATION OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1967 Sep;58(3):946–953. doi: 10.1073/pnas.58.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M., Sueoka N. Location of genetic loci of ribosomal RNA on Bacillus subtilis chromosome. Proc Natl Acad Sci U S A. 1965 Aug;54(2):483–491. doi: 10.1073/pnas.54.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosset R., Julien J., Monier R. Ribonucleic acid composition of bacteria as a function of growth rate. J Mol Biol. 1966 Jul;18(2):308–320. doi: 10.1016/s0022-2836(66)80248-6. [DOI] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. I. Ordered enzyme synthesis. J Bacteriol. 1968 Feb;95(2):469–478. doi: 10.1128/jb.95.2.469-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan T. A., Thach R. E. Role of the formylmethionine codon AUG in phasing translation of synthetic messenger RNA. J Mol Biol. 1966 Aug;19(1):74–90. doi: 10.1016/s0022-2836(66)80051-7. [DOI] [PubMed] [Google Scholar]
- Torriani A., Levinthal C. Ordered synthesis of proteins during outgrowth of spores of Bacillus cereus. J Bacteriol. 1967 Jul;94(1):176–183. doi: 10.1128/jb.94.1.176-183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARY J. C., HALVORSON H. O. KINETICS OF GERMINATION OF BACILLUS SPORES. J Bacteriol. 1965 May;89:1340–1347. doi: 10.1128/jb.89.5.1340-1347.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinter V., Slepecky R. A. Direct Transition of Outgrowing Bacterial Spores to New Sporangia Without Intermediate Cell Division. J Bacteriol. 1965 Sep;90(3):803–807. doi: 10.1128/jb.90.3.803-807.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESE C. R. Unusual ribosome particles occurring during spore germination. J Bacteriol. 1961 Nov;82:695–701. doi: 10.1128/jb.82.5.695-701.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]


