Figure 1. CNF1 effects on bladder cell motility and bacterial entry.
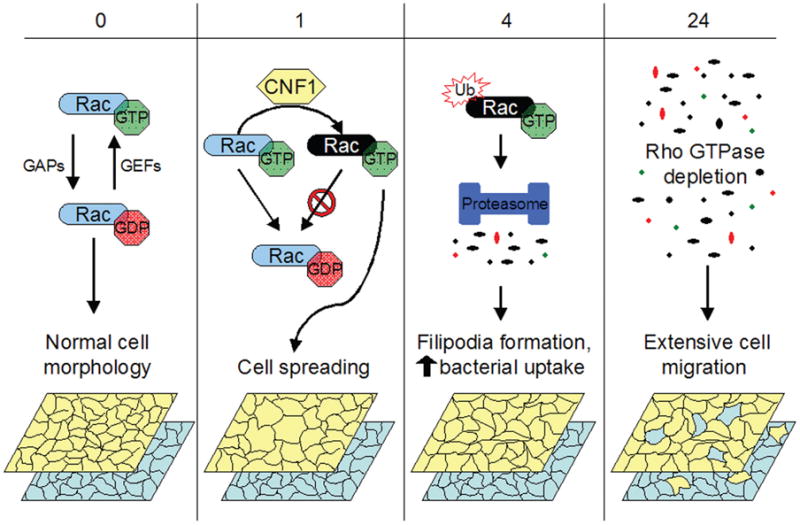
In normal uninfected bladder epithelial cells, Rho GTPases like Rac cycle between active GTP-bound and inactive GDP-bound states, regulated by GAPs and GEFs (guanine nucleotide exchange factors). CNF1 renders Rho GTPases constitutively active by catalyzing deamidation of glutamine 63 in RhoA (or glutamine 61 in Rac and Cdc42). CNF1-modified constitutively active Rac, in this example, is shown as a black oval. Activation of Rac and other Rho GTPases leads to cytoskeletal rearrangements resulting in increased cell spreading and membrane ruffling (1–4 h post-CNF1 intoxication). By 4 h postintoxication, constitutively activated Rho GTPases are ubiquitinated and targeted for proteasomal degradation. The resulting depletion of Rho GTPases promotes host cytoskeletal rearrangements, resulting in enhanced filopodia formation and stimulating bacterial uptake. Bacterial internalization continues to increase up to 24 h post-CNF1 intoxication. Rho depletion also stimulates bladder cell migration. Theoretically, this could result in redistribution of host cells within a stratified three-dimensional tissue such as the bladder epithelium, allowing UPEC to more efficiently disseminate and colonize underlying cells. The model shows the migration of host cells in the upper (yellow) and lower (blue) layers of an idealized tissue bilayer as a consequence of CNF1-stimulated Rac depletion.
