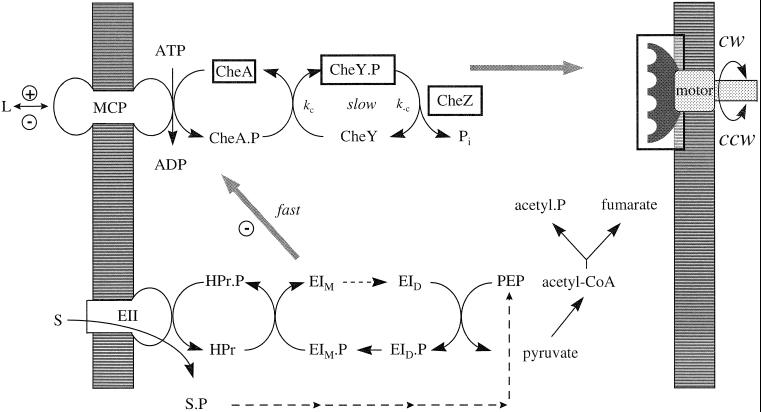
Figure 7.
Properties of the PTS chemotactic pathway. The PTS is poised far from equilibrium because of the slow dimerization of EI monomers (EID to EIM, dashed arrow), obligatory for phosphorylation by PEP. The positive chemotactic signal triggered by transport of PTS substrates (S) may originate either from a decrease in phosphorylation levels of the donor phosphorelay or from changes in metabolite levels caused by PEP consumption. It may act on CheA, CheZ, CheY.P, or the motor switch (boxed items). The present study sought to distinguish between these possibilities. The analysis of mutant responses showed that the PTS signal acted solely to inhibit MCP-bound CheA. The high response sensitivity argued that it is generated by the dephosphorylation of PTS phosphorelay components, most likely EI. PEP levels decrease little, and their subsequent restoration by metabolism of d-glc (dashed arrows, small arrowheads) does not affect signaling. The response kinetics implied that signaling (gray arrows) was limited, as for periplasmic MCP ligands (L), by CheY.P dephosphorylation (i.e., kc + k−c) over most of the PTS chemotactic response range if PTS dephosphorylation kinetics were determined by the transport rate, consistent with known transport and biochemical data. Rephosphorylation of the PTS phosphorelay on withdrawal of PTS substrates will be limited by EI dimerization and may not be rapid enough to stimulate CheA (+), in contrast to withdrawal of MCP attractant ligands. Evidence for such a signal was not obtained.