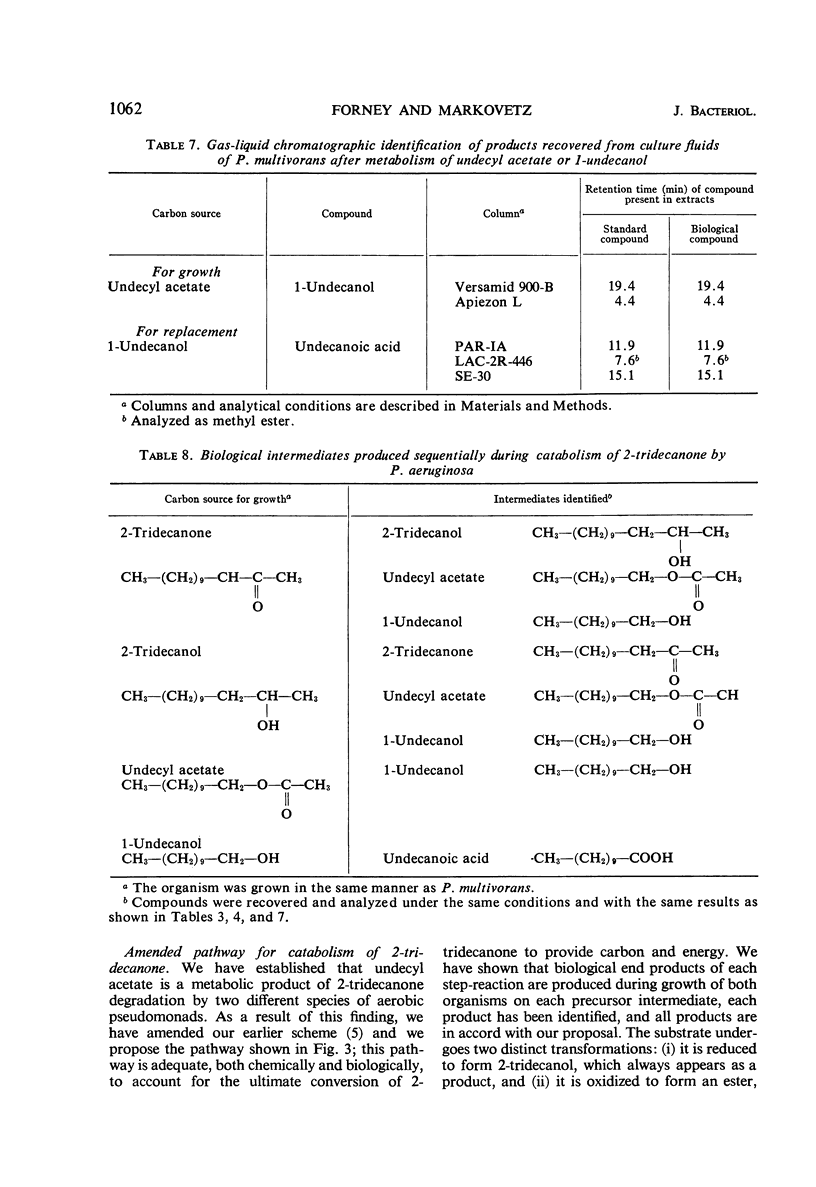
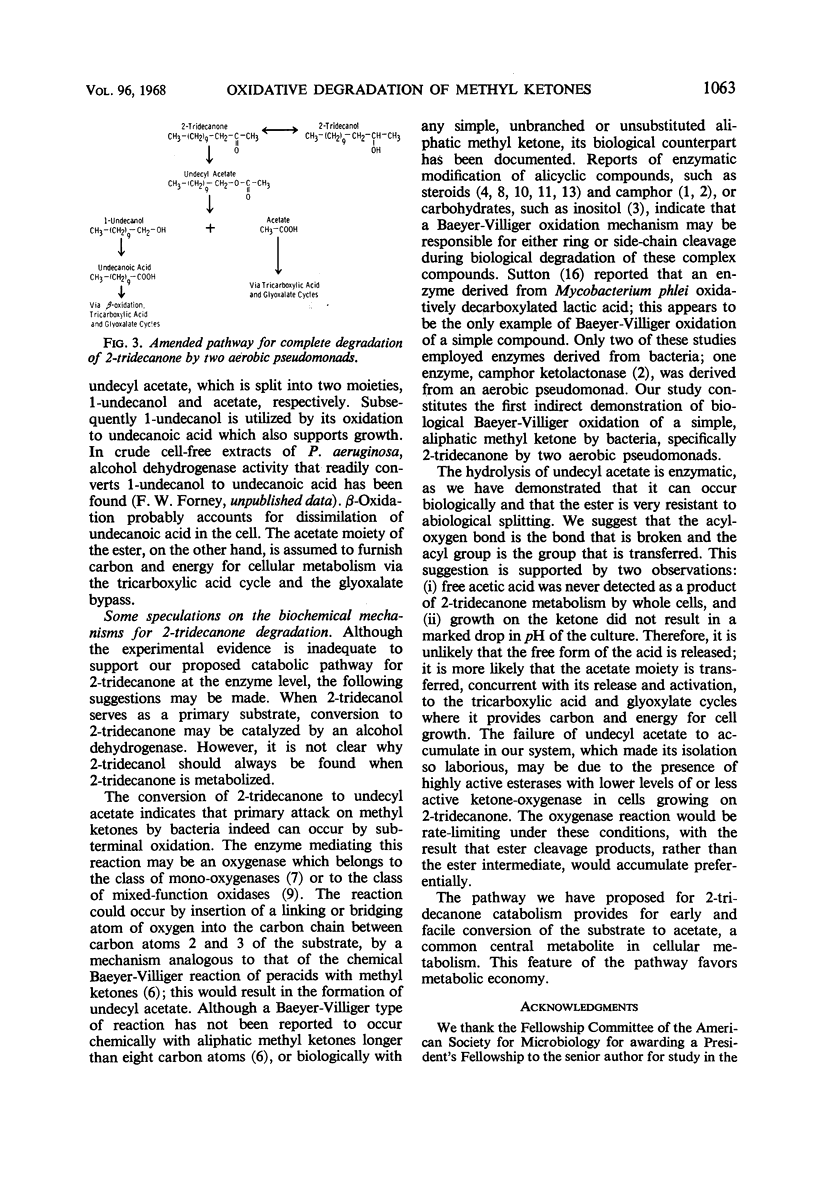
Abstract
A new intermediate was identified in the 2-tridecanone pathway of Pseudomonas multivorans, formerly designated pseudomonad 4G-9. This intermediate, undecyl acetate, was isolated directly from growing cultures of the organism; the structure of the intermediate was determined by infrared spectroscopy and by gas-liquid chromatographic identification of its hydrolytic products. An amended pathway is presented that accounts for the conversion of 2-tridecanone to provide carbon and energy for growth. It was shown that all early intermediates in the pathway arise biologically and sequentially from their precursors. Studies with P. aeruginosa showed that this organism also degrades 2-tridecanone by the pathway characteristic of P. multivorans. Biochemical mechanisms of the pathway are discussed. Discovery of undecyl acetate confirms our earlier contention that the primary attack on methyl ketones by bacteria can be by subterminal oxidation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHARALAMPOUS F. C. Biochemical studies on inositol. VI. Mechanism of cleavage of inositol to D-glucuronic acid. J Biol Chem. 1960 May;235:1286–1291. [PubMed] [Google Scholar]
- CONRAD H. E., DUBUS R., GUNSALUS I. C. An enzyme system for cyclic ketone lactonization. Biochem Biophys Res Commun. 1961 Nov 29;6:293–297. doi: 10.1016/0006-291x(61)90382-5. [DOI] [PubMed] [Google Scholar]
- CONRAD H. E., DUBUS R., NAMTVEDT M. J., GUNSALUS I. C. MIXED FUNCTION OXIDATION. II. SEPARATION AND PROPERTIES OF THE ENZYMES CATALYZING CAMPHOR LACTONIZATION. J Biol Chem. 1965 Jan;240:495–503. [PubMed] [Google Scholar]
- Forney F. W., Markovetz A. J., Kallio R. E. Bacterial oxidation of 2-tridecanone to 1-undecanol. J Bacteriol. 1967 Feb;93(2):649–655. doi: 10.1128/jb.93.2.649-655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASKIN A. I., GRABOWICH P., LISLEMEYERSC DE, FRIED J. TRANSFORMATIONS OF EBURICOIC ACID. V. CLEAVAGE OF RING A BY THE FUNGUS GLOMERELLA FUSARIOIDES. J Med Chem. 1964 Jul;7:406–409. doi: 10.1021/jm00334a003. [DOI] [PubMed] [Google Scholar]
- MASON H. S. Mechanisms of oxygen metabolism. Adv Enzymol Relat Subj Biochem. 1957;19:79–233. doi: 10.1002/9780470122648.ch2. [DOI] [PubMed] [Google Scholar]
- PRAIRIE R. L., TALALAY P. Enzymatic formation of testololactone. Biochemistry. 1963 Jan-Feb;2:203–208. doi: 10.1021/bi00901a039. [DOI] [PubMed] [Google Scholar]
- RADIN N. S., HAJRA A. K., AKAHORI Y. Preparation of methyl esters. J Lipid Res. 1960 Apr;1:250–251. [PubMed] [Google Scholar]
- Rahm M. A., Sih C. J. Mechanisms of steroid oxidation by microorganisms. XI. Enzymatic cleavage of the pregnane side chain. J Biol Chem. 1966 Aug 10;241(15):3615–3623. [PubMed] [Google Scholar]
- SUTTON W. B. Mechanism of action and crystallization of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1957 May;226(1):395–405. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]


