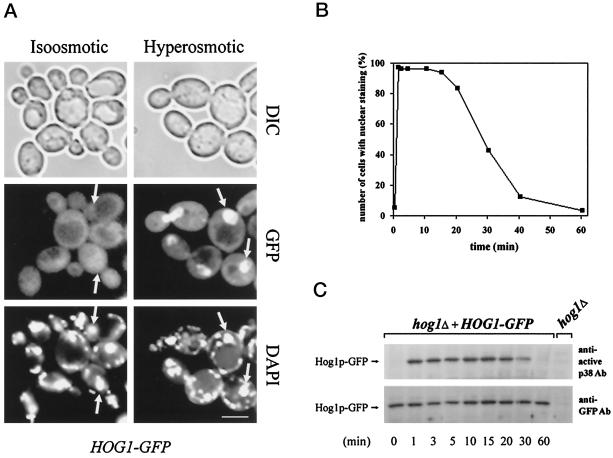
Figure 2.
Hog1–GFP nuclear accumulation in response to hy-perosmotic stress correlates with its dual phosphorylation status. (A) Hyperosmotic stress induces nuclear accumulation of Hog1. Logarithmically growing strain K4327 (hog1Δ) transformed with pVR65–WT (HOG1–GFP) was examined by fluorescence or light microscopy under iso-osmotic (selective medium) or hyperosmotic growth conditions (5 min after addition of NaCl into selective medium to a final concentration of 0.4 M). In agreement with the observations of others (Stade et al., 1997), DAPI used for staining of nuclei preferentially stains mitochondrial DNA in living cells. Positions of nuclei are indicated by arrows. DIC, differential interference contrast. Bar, 5 μm. (B) The kinetics of Hog1 nuclear accumulation and dual phosphorylation are similar. Strain K4327 (hog1Δ) was transformed with centromer plasmid pVR65–WT (HOG1–GFP). Cells were grown in selective medium to logarithmic phase (iso-osmotic conditions). A control sample of unstressed cells was taken (time 0 min) followed by addition of NaCl to a final concentration of 0.4 M. Then cells were incubated for various times and examined by fluorescence microscopy. To determine the activation profile of Hog1–GFP, samples were taken at the time points indicated, and protein extracts were prepared. Western blots were analyzed with anti-active p38 MAPK antibody and with anti-GFP antibody (C). In a control experiment, no band corresponding to the position of the Hog1–GFP-specific band was detected with anti-GFP antibody when protein extract from hog1Δ strain was used.