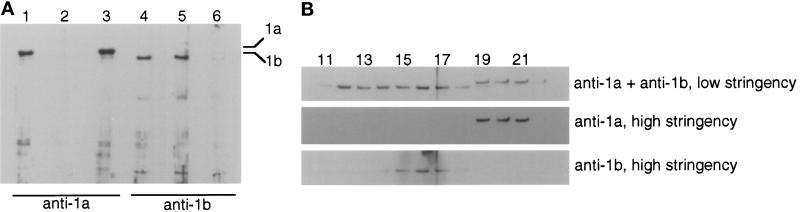
Figure 6.
Western blot analysis of sucrose gradient fractions under high-stringency conditions. (A) Western blots using antibodies absorbed with synthetic peptides. Testis cytoplasmic dynein was electrophoresed in 6.7% SDS-polyacrylamide gels, transferred to nitrocellulose, and probed with affinity-purified antibodies that had been preabsorbed with synthetic peptides. The Western blots were processed using the high-stringency conditions (see MATERIALS AND METHODS). Anti-1a antibody: lane 1, no absorption; lane 2, preabsorbed with 1a peptide; lane 3, preabsorbed with 1b peptide. Anti-1b antibody: lane 4, no absorption; lane 5, preabsorbed with 1a peptide; lane 6, preabsorbed with 1b peptide. Under these conditions, the antibodies reacted with the appropriate heavy chain wholly in a sequence-specific manner. (B) Western blots of sucrose gradient fractions. Selected fractions of a low-ionic strength sucrose gradient similar to the one shown in Figure 5 were electrophoresed, blotted, and probed with antibodies. The same blot was repeatedly stripped and reprobed. The heavy chain regions of the blots are shown. Top, anti-1b at low stringency, to which anti-1a was added. Middle, anti-1a at high stringency. Bottom, anti-1b at high stringency. The anti-1b antibody reacted only with a subset of the 1b-like proteins present in fractions 11–17 (see Figure 5).