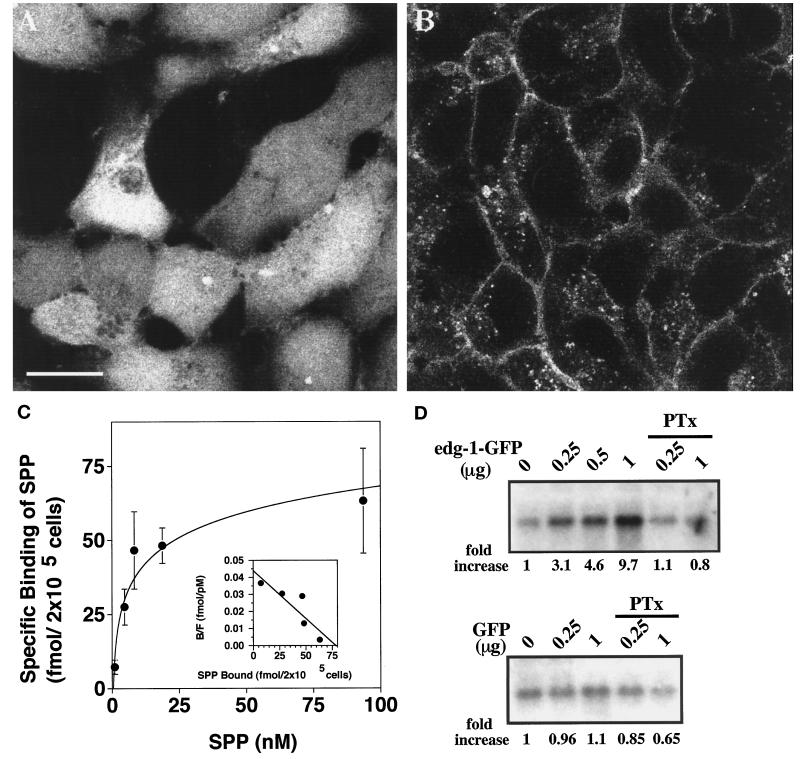
Figure 1.
Characterization of the EDG-1–GFP fusion protein. (A and B) Subcellular localization of GFP and EDG-1–GFP polypeptides is shown. HEK293 cells stably transfected with GFP (A) or EDG-1–GFP (B) were visualized by confocal fluorescence microscopy. Note that the GFP polypeptide is primarily cytosolic, whereas the EDG-1–GFP polypeptide is localized on the plasma membrane and intracellular vesicles. Bar, 10 μm. (C) EDG-1–GFP binds to SPP. HEK293 cells stably transfected with the EDG-1–GFP construct were used in whole-cell binding assays with [32P]SPP as described (Lee et al., 1998b). Data represent the mean of triplicate determinations of specific binding: (total − nonspecific binding) in 2 × 105 cells. Inset, Scatchard analysis of the binding isotherm indicates a Kd of 7.4 nM and a Bmax of 77 fmol per 2 × 105 cells. (D) EDG-1–GFP–dependent MAP kinase activation by SPP is shown. Cos-1 cells were transiently cotransfected with different amounts of EDG-1–GFP or GFP plasmids and a fixed amount of HA–ERK-2 plasmid. Total DNA input was kept constant with vector DNA. Thirty hours after transfection, cells were starved for 16 h and then stimulated with 50 nM SPP for 2 min. Cell lysates were prepared, and ERK-2 activity was measured using the immune complex kinase assay as described. Autoradiographs of the phosphorylated substrate, myelin basic protein, are shown. Quantitative data derived from densitometric scans are shown below the autoradiographs (fold increase). Some cultures received pertussis toxin (PTx) (400 ng/ml) for 3 h before SPP stimulation.