Abstract
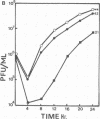
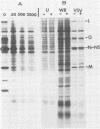
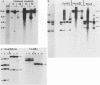
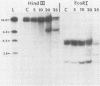
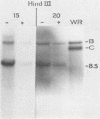
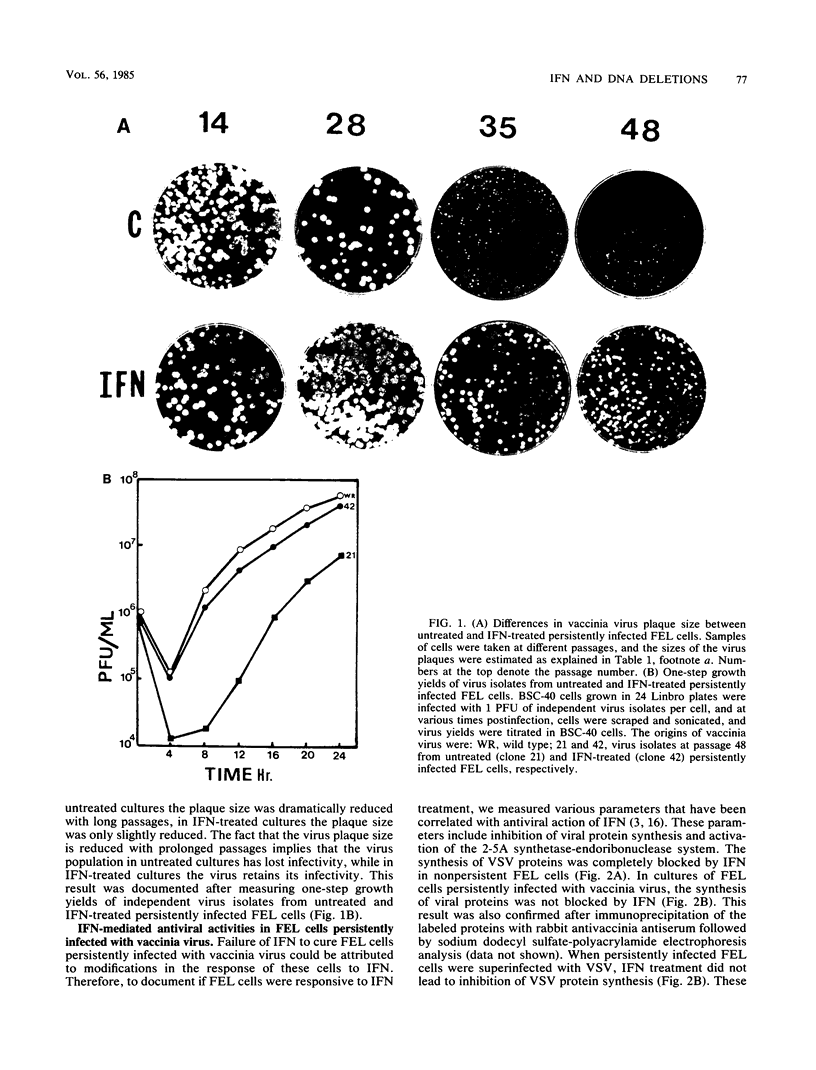
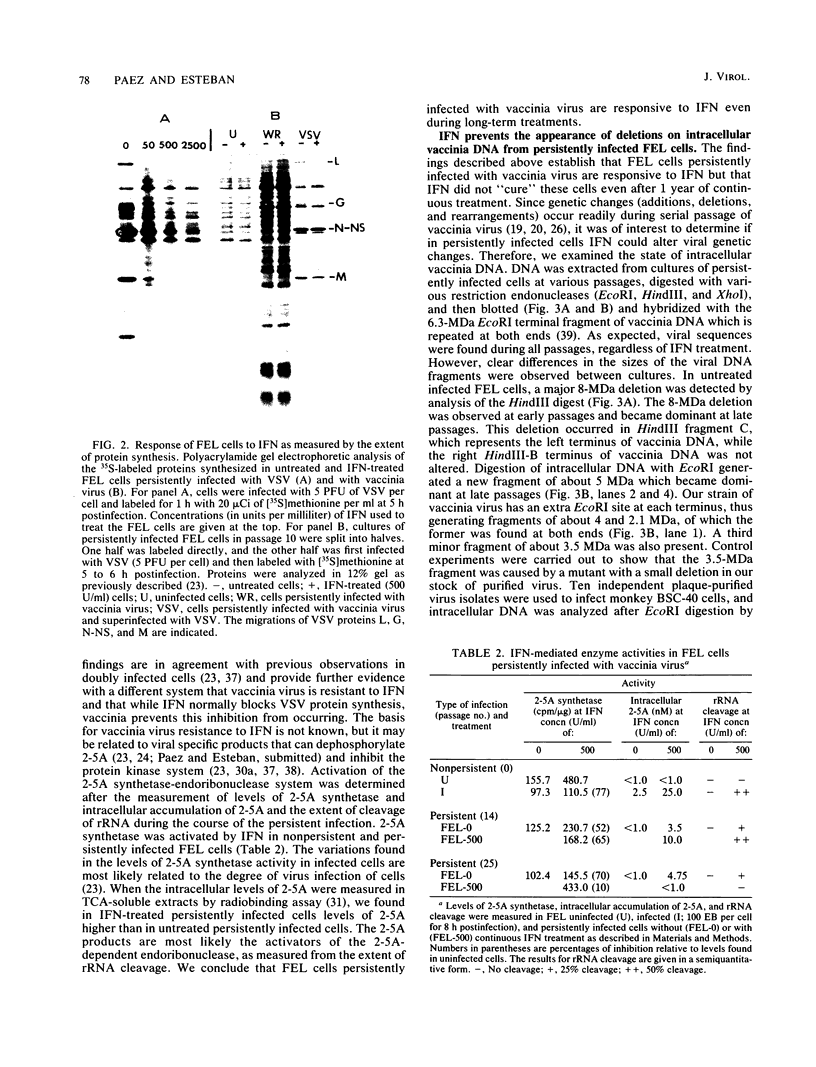
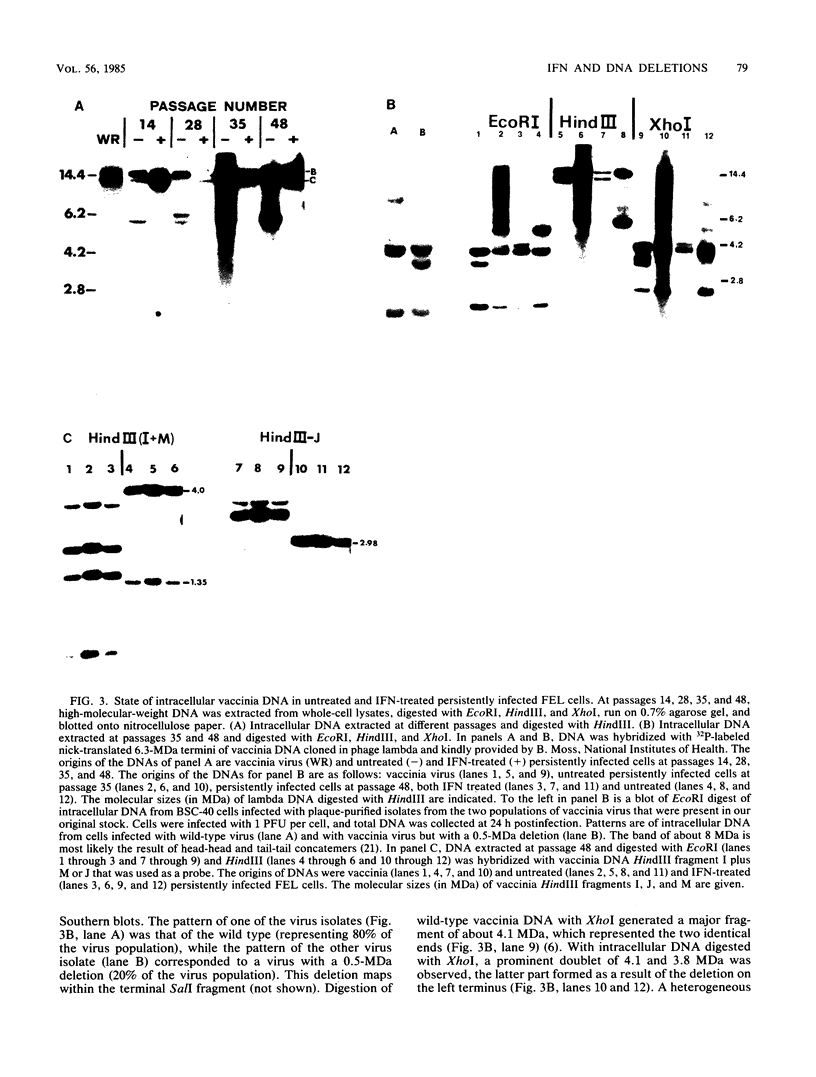
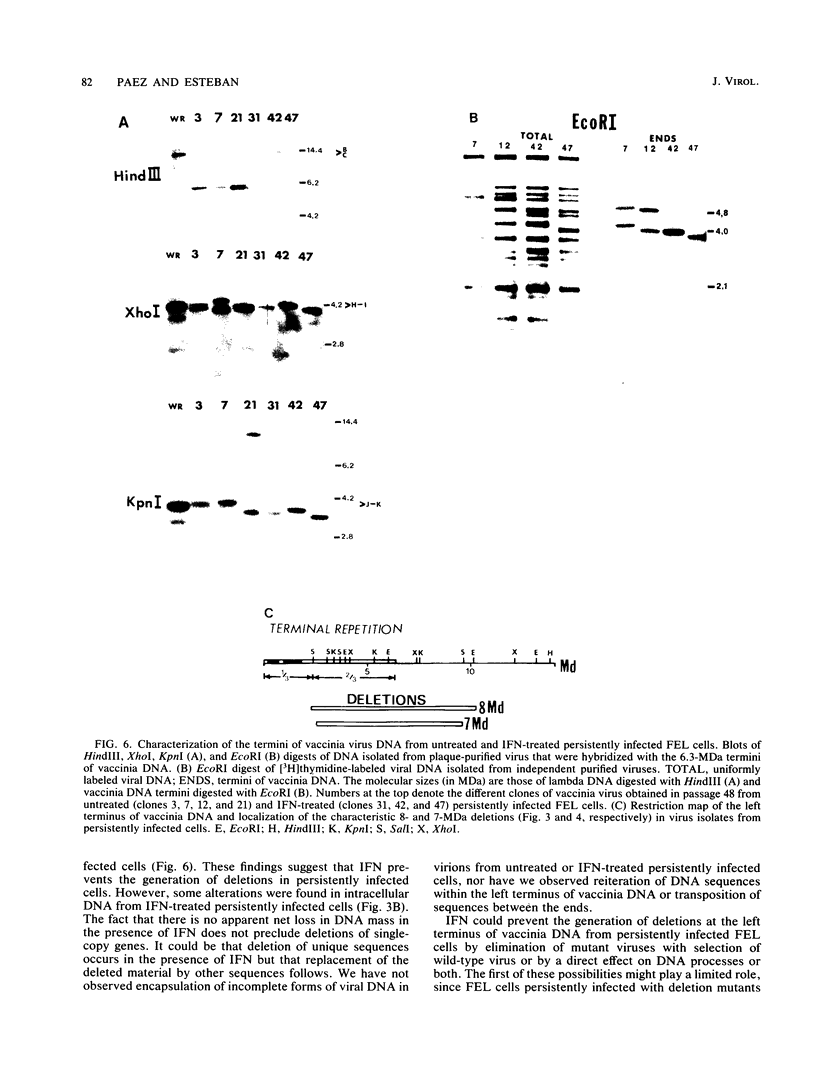
In this report we have shown that Friend erythroleukemia cells persistently infected with vaccinia virus maintain the persistent infection even after 1 year of continuous interferon (IFN) treatment. The persistently infected cultures were responsive to IFN as determined by their ability to induce 2-5A synthetase, to increase the intracellular levels of 2-5A, and to cause rRNA cleavage. While large deletions at the left terminus of vaccinia DNA occurred readily in the virus population from untreated cells, IFN completely suppressed the generation of these spontaneous deletions. Removal of IFN from these cultures led to the appearance of similar deletions at the left terminus of the viral genome. The regions deleted contain more than half of the left-end inverted terminal repetition of the vaccinia genome. These findings show that IFN alters specific events associated with the generation of vaccinia DNA deletions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Archard L. C., Mackett M., Barnes D. E., Dumbell K. R. The genome structure of cowpox virus white pock variants. J Gen Virol. 1984 May;65(Pt 5):875–886. doi: 10.1099/0022-1317-65-5-875. [DOI] [PubMed] [Google Scholar]
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Boni C., Esteban M., Pellicer A. Expression of cloned vaccinia virus DNA sequences introduced into animal cells. J Gen Virol. 1984 Jul;65(Pt 7):1245–1251. doi: 10.1099/0022-1317-65-7-1245. [DOI] [PubMed] [Google Scholar]
- DeFilippes F. M. Restriction enzyme mapping of vaccinia virus DNA. J Virol. 1982 Jul;43(1):136–149. doi: 10.1128/jvi.43.1.136-149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M. F., Vignal M., Le Cunff M., Chany C. Interferon inhibits transformation of mouse cells by exogenous cellular or viral genes. Nature. 1983 Jun 2;303(5916):433–435. doi: 10.1038/303433a0. [DOI] [PubMed] [Google Scholar]
- Esposito J. J., Cabradilla C. D., Nakano J. H., Obijeski J. F. Intragenomic sequence transposition in monkeypox virus. Virology. 1981 Mar;109(2):231–243. doi: 10.1016/0042-6822(81)90495-5. [DOI] [PubMed] [Google Scholar]
- Esteban M. Analysis of replicating vaccinia DNA in interferon-treated, virus-infected cells. J Interferon Res. 1984 Spring;4(2):179–192. doi: 10.1089/jir.1984.4.179. [DOI] [PubMed] [Google Scholar]
- Esteban M., Benavente J., Paez E. Effect of interferon on integrity of vaccinia virus and ribosomal RNA in infected cells. Virology. 1984 Apr 15;134(1):40–51. doi: 10.1016/0042-6822(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Esteban M. Defective vaccinia virus particles in interferon-treated infected cells. Virology. 1984 Feb;133(1):220–227. doi: 10.1016/0042-6822(84)90443-4. [DOI] [PubMed] [Google Scholar]
- Esteban M., Paez E. Antiviral and antiproliferative properties of interferons: mechanism of action. Prog Med Virol. 1985;32:159–173. [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Gewert D. R., Shah S., Clemens M. J. Inhibition of cell division by interferons: Changes in the transport and intracellular metabolism of thymidine in human lymphoblastoid (Daudi) cells. Eur J Biochem. 1981 Jun 1;116(3):487–492. doi: 10.1111/j.1432-1033.1981.tb05362.x. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Greenberg B., Lacks S. A. Generation of deletions in pneumococcal mal genes cloned in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5189–5193. doi: 10.1073/pnas.81.16.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G., Gewert D. R., Clemens M. J. Inhibition of cell proliferation by interferons. 2. Changes in processing and stability of newly synthesized DNA in human lymphoblastoid (Daudi) cells. Eur J Biochem. 1984 Mar 15;139(3):627–635. doi: 10.1111/j.1432-1033.1984.tb08050.x. [DOI] [PubMed] [Google Scholar]
- Moss B., Winters E., Cooper J. A. Deletion of a 9,000-base-pair segment of the vaccinia virus genome that encodes nonessential polypeptides. J Virol. 1981 Nov;40(2):387–395. doi: 10.1128/jvi.40.2.387-395.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Winters E., Cooper N. Instability and reiteration of DNA sequences within the vaccinia virus genome. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1614–1618. doi: 10.1073/pnas.78.3.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer R. W., Graves R. L. The mechanism of cytoplasmic orthopoxvirus DNA replication. Cell. 1981 Dec;27(2 Pt 1):391–401. doi: 10.1016/0092-8674(81)90422-0. [DOI] [PubMed] [Google Scholar]
- Paez E., Esteban M. Interferon inhibits marker rescue of vaccinia virus. J Interferon Res. 1985 Spring;5(2):247–256. doi: 10.1089/jir.1985.5.247. [DOI] [PubMed] [Google Scholar]
- Paez E., Esteban M. Nature and mode of action of vaccinia virus products that block activation of the interferon-mediated ppp(A2'p)nA-synthetase. Virology. 1984 Apr 15;134(1):29–39. doi: 10.1016/0042-6822(84)90269-1. [DOI] [PubMed] [Google Scholar]
- Paez E., Esteban M. Resistance of vaccinia virus to interferon is related to an interference phenomenon between the virus and the interferon system. Virology. 1984 Apr 15;134(1):12–28. doi: 10.1016/0042-6822(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Panicali D., Davis S. W., Mercer S. R., Paoletti E. Two major DNA variants present in serially propagated stocks of the WR strain of vaccinia virus. J Virol. 1981 Mar;37(3):1000–1010. doi: 10.1128/jvi.37.3.1000-1010.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Esteban M. Gene-transfer, stability, and biochemical properties of animal cells transformed with vaccinia DNA. Virology. 1982 Oct 30;122(2):363–380. doi: 10.1016/0042-6822(82)90236-7. [DOI] [PubMed] [Google Scholar]
- Perucho M., Esteban M. Inhibitory effect of interferon on the genetic and oncogenic transformation by viral and cellular genes. J Virol. 1985 Apr;54(1):229–232. doi: 10.1128/jvi.54.1.229-232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup D. J., Ink B. S., Parsons B. L., Hu W., Joklik W. K. Spontaneous deletions and duplications of sequences in the genome of cowpox virus. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6817–6821. doi: 10.1073/pnas.81.21.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Friend C. Persistent infection of Friend erythroleukemia cells with vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4805–4809. doi: 10.1073/pnas.79.15.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Kerr I. M. Interferon-mediated, double-stranded RNA-dependent protein kinase is inhibited in extracts from vaccinia virus-infected cells. J Virol. 1984 Apr;50(1):229–236. doi: 10.1128/jvi.50.1.229-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Roberts W. K., Kerr I. M. 2-5A accumulates to high levels in interferon-treated, vaccinia virus-infected cells in the absence of any inhibition of virus replication. J Virol. 1984 Apr;50(1):220–228. doi: 10.1128/jvi.50.1.220-228.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R. H., Watling D., Balkwill F. R., Trowsdale J., Kerr I. M. The ppp(A2'p)nA and protein kinase systems in wild-type and interferon-resistant Daudi cells. Eur J Biochem. 1982 Aug;126(2):333–341. doi: 10.1111/j.1432-1033.1982.tb06783.x. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Weil J., Epstein C. J., Epstein L. B., Sedmak J. J., Sabran J. L., Grossberg S. E. A unique set of polypeptides is induced by gamma interferon in addition to those induced in common with alpha and beta interferons. Nature. 1983 Feb 3;301(5899):437–439. doi: 10.1038/301437a0. [DOI] [PubMed] [Google Scholar]
- Whitaker-Dowling P., Youngner J. S. Characterization of a specific kinase inhibitory factor produced by vaccinia virus which inhibits the interferon-induced protein kinase. Virology. 1984 Aug;137(1):171–181. doi: 10.1016/0042-6822(84)90020-5. [DOI] [PubMed] [Google Scholar]
- Whitaker-Dowling P., Youngner J. S. Vaccinia rescue of VSV from interferon-induced resistance: reversal of translation block and inhibition of protein kinase activity. Virology. 1983 Nov;131(1):128–136. doi: 10.1016/0042-6822(83)90539-1. [DOI] [PubMed] [Google Scholar]
- Wittek R., Barbosa E., Cooper J. A., Garon C. F., Chan H., Moss B. Inverted terminal repetition in vaccinia virus DNA encodes early mRNAs. Nature. 1980 May 1;285(5759):21–25. doi: 10.1038/285021a0. [DOI] [PubMed] [Google Scholar]
- Wittek R., Moss B. Tandem repeats within the inverted terminal repetition of vaccinia virus DNA. Cell. 1980 Aug;21(1):277–284. doi: 10.1016/0092-8674(80)90135-x. [DOI] [PubMed] [Google Scholar]