Figure 1.
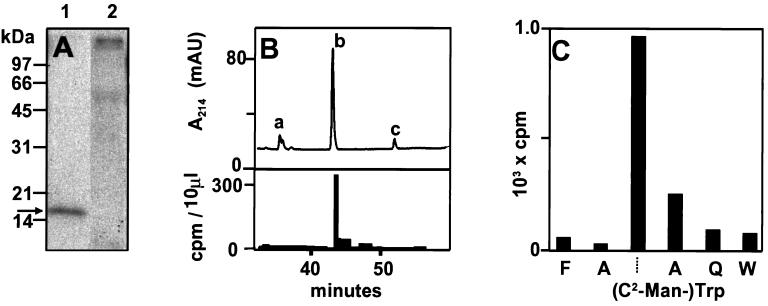
Characterization of RNase 2.4 labeled with D-[2-3H]Mannose. (A) NIH 3T3 cells were transfected with the plasmid encoding RNase 2.4 and labeled with D-[2-3H]mannose. RNase 2.4 was immunoprecipitated with polyclonal α-RNase 4 antibodies from 6.5 ml of conditioned medium, and fractionated on a 12.5% SDS-PAA gel (lane 1). The immunoprecipitate of conditioned medium from nontransfected control cells was loaded in lane 2. The gel was submitted to fluorography and exposed for 4 d at −70°C. The position of RNase 4, which was visualized by Coomasssie brilliant blue staining, has been indicated by an arrow. (B) Portion of the thermolytic peptide map of radiolabeled RNase 2.4. Tritiated RNase 2.4 was purified from conditioned medium from transfected 3T3 cells and mixed with 1.2 μg of r-RNase 2/E-coli and 1.2 μg of RNase 2/urine. The mixture was digested with thermolysin at 75°C and the peptides were fractionated by C18 reversed phase HPLC. Fractions were collected and examined for radioactivity. The width of the bar indicates the fraction size and the height represents the radioactivity in 10 μl. The peaks containing unmodified peptides 6–10 (TWAQW) and 5–10 (FTWAQW) have been labeled ‘a’ and ‘c’, respectively. C-mannosylated peptide 5–10 has been labeled with ‘b’. (C) Solid-phase Edman degradation of peptide 5–10. Radioactive peptide from ‘B’ was covalently coupled to a sequelon-AA membrane and sequenced. The radioactivity of the anilinothiazolinone-amino acid released at each cycle was determined.