Abstract
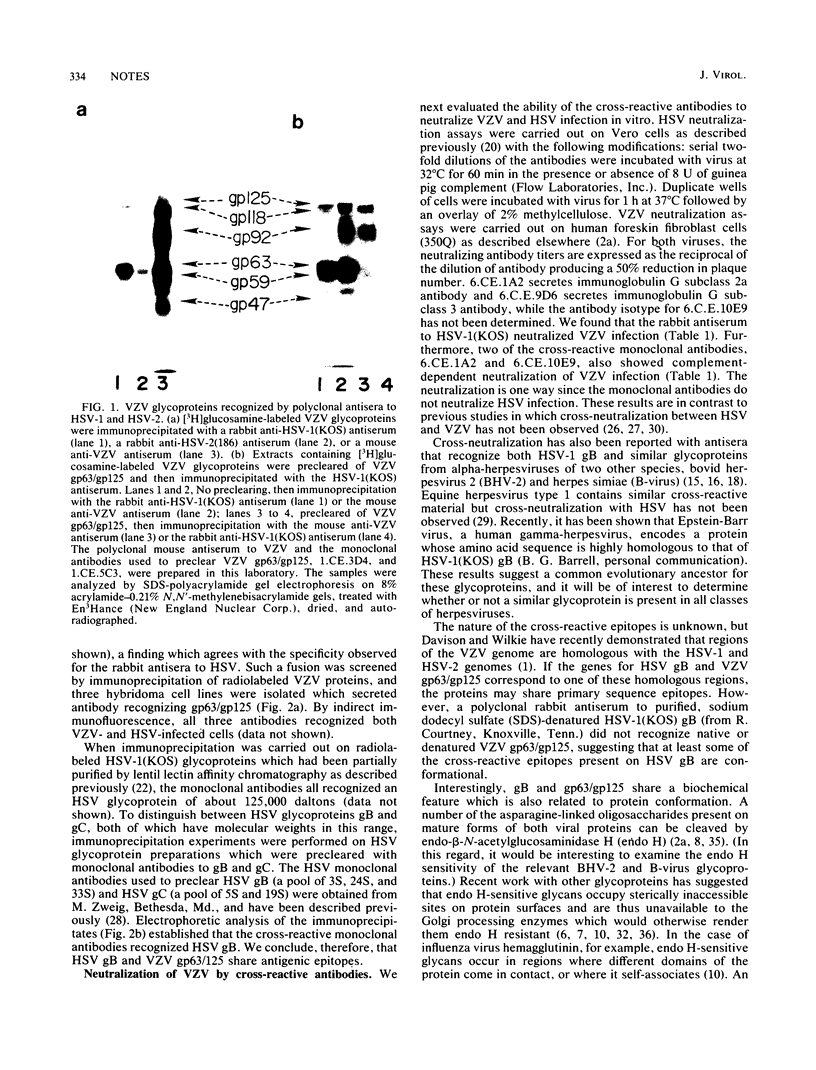
Cross-reactive monoclonal antibodies recognizing both herpes simplex virus (HSV) glycoprotein B and a major 63,000-dalton varicella-zoster virus (VZV) envelope glycoprotein were isolated and found to neutralize VZV infection in vitro. None of the other VZV glycoproteins was recognized by any polyclonal anti-HSV serum tested. These results demonstrate that HSV glycoprotein B and the 63,000-dalton VZV glycoprotein share antigenic epitopes and raise the possibility that these two proteins have a similar function in infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davison A. J., Wilkie N. M. Location and orientation of homologous sequences in the genomes of five herpesviruses. J Gen Virol. 1983 Sep;64(Pt 9):1927–1942. doi: 10.1099/0022-1317-64-9-1927. [DOI] [PubMed] [Google Scholar]
- DeLuca N., Bzik D. J., Bond V. C., Person S., Snipes W. Nucleotide sequences of herpes simplex virus type 1 (HSV-1) affecting virus entry, cell fusion, and production of glycoprotein gb (VP7). Virology. 1982 Oct 30;122(2):411–423. doi: 10.1016/0042-6822(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Edson C. M., Hosler B. A., Poodry C. A., Schooley R. T., Waters D. J., Thorley-Lawson D. A. Varicella-zoster virus envelope glycoproteins: biochemical characterization and identification in clinical material. Virology. 1985 Aug;145(1):62–71. doi: 10.1016/0042-6822(85)90201-6. [DOI] [PubMed] [Google Scholar]
- Forghani B., Dupuis K. W., Schmidt N. J. Varicella-zoster viral glycoproteins analyzed with monoclonal antibodies. J Virol. 1984 Oct;52(1):55–62. doi: 10.1128/jvi.52.1.55-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Friedrichs W. E., Weigle K. A., McGuire W. L. Monoclonal antibodies against three major glycoproteins of varicella-zoster virus. Infect Immun. 1983 Apr;40(1):381–388. doi: 10.1128/iai.40.1.381-388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Weigle K. A., Friedrichs W. E., McGuire W. L. Varicella-zoster virus-specific gp140: a highly immunogenic and disulfide-linked structural glycoprotein. Virology. 1984 Jan 15;132(1):138–146. doi: 10.1016/0042-6822(84)90098-9. [DOI] [PubMed] [Google Scholar]
- Hsieh P., Robbins P. W. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984 Feb 25;259(4):2375–2382. [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Selective cleavage by endo-beta-N-acetylglucosaminidase H at individual glycosylation sites of Sindbis virion envelope glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2555–2561. [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Keil W., Niemann H., Schwarz R. T., Klenk H. D. Carbohydrates of influenza virus. V. Oligosaccharides attached to individual glycosylation sites of the hemagglutinin of fowl plague virus. Virology. 1984 Feb;133(1):77–91. doi: 10.1016/0042-6822(84)90427-6. [DOI] [PubMed] [Google Scholar]
- Keller P. M., Neff B. J., Ellis R. W. Three major glycoprotein genes of varicella-zoster virus whose products have neutralization epitopes. J Virol. 1984 Oct;52(1):293–297. doi: 10.1128/jvi.52.1.293-297.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Little S. P., Jofre J. T., Courtney R. J., Schaffer P. A. A virion-associated glycoprotein essential for infectivity of herpes simplex virus type 1. Virology. 1981 Nov;115(1):149–160. doi: 10.1016/0042-6822(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Norrild B., Ludwig H., Rott R. Identification of a common antigen of herpes simplex virus bovine herpes mammillitis virus, and B virus. J Virol. 1978 Jun;26(3):712–717. doi: 10.1128/jvi.26.3.712-717.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T., Yamanishi K., Shiraki K., Takahashi M. Synthesis and processing of glycoproteins of Varicella-Zoster virus (VZV) as studied with monoclonal antibodies to VZV antigens. Virology. 1983 Sep;129(2):357–368. doi: 10.1016/0042-6822(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Pancake B. A., Aschman D. P., Schaffer P. A. Genetic and phenotypic analysis of herpes simplex virus type 1 mutants conditionally resistant to immune cytolysis. J Virol. 1983 Sep;47(3):568–585. doi: 10.1128/jvi.47.3.568-585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue-Geile K. L., Lee G. T., Shapira S. K., Spear P. G. Fine mapping of mutations in the fusion-inducing MP strain of herpes simplex virus type 1. Virology. 1984 Jul 15;136(1):100–109. doi: 10.1016/0042-6822(84)90251-4. [DOI] [PubMed] [Google Scholar]
- Respess R. A., Pancake B. A., Edson C. M., Schaffer P. A. A rapid procedure for the enrichment of undenaturated, antigenically active herpes simplex virus glycoproteins. J Virol Methods. 1984 Feb;8(1-2):27–45. doi: 10.1016/0166-0934(84)90038-7. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J. Further evidence for common antigens in herpes simplex and varicella-zoster viruses. J Med Virol. 1982;9(1):27–36. doi: 10.1002/jmv.1890090105. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Neutralizing antibody responses to varicella-zoster virus. Infect Immun. 1975 Sep;12(3):606–613. doi: 10.1128/iai.12.3.606-613.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki K., Okuno T., Yamanishi K., Takahashi M. Polypeptides of varicella-zoster virus (VZV) and immunological relationship of VZV and herpes simplex virus (HSV). J Gen Virol. 1982 Aug;61(Pt 2):255–269. doi: 10.1099/0022-1317-61-2-255. [DOI] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden B. W., Kinchington P. R., Powell K. L., Halliburton I. W. Antigenic and biochemical analysis of gB of herpes simplex virus type 1 and type 2 and of cross-reacting glycoproteins induced by bovine mammillitis virus and equine herpesvirus type 1. J Gen Virol. 1985 Feb;66(Pt 2):231–247. doi: 10.1099/0022-1317-66-2-231. [DOI] [PubMed] [Google Scholar]
- Svedmyr A. Varicella virus in HeLa cells. Arch Gesamte Virusforsch. 1965;17(3):495–503. doi: 10.1007/BF01241206. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Edson C. M. Polypeptides of the Epstein-Barr virus membrane antigen complex. J Virol. 1979 Nov;32(2):458–467. doi: 10.1128/jvi.32.2.458-467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Chu F. K. GlycoProtein biosynthesis in yeast. protein conformation affects processing of high mannose oligosaccharides on carboxypeptidase Y and invertase. J Biol Chem. 1983 Feb 25;258(4):2562–2567. [PubMed] [Google Scholar]
- Trlifajová J., Sourek J., Ryba M. Antigenic relationship between varicella-herpes zoster and herpes simplex viruses studied by the gel precipitation reaction. Acta Virol. 1971 Jul;15(4):293–300. [PubMed] [Google Scholar]
- Vafai A., Wroblewska Z., Wellish M., Green M., Gilden D. Analysis of three late varicella-zoster virus proteins, a 125,000-molecular-weight protein and gp1 and gp3. J Virol. 1984 Dec;52(3):953–959. doi: 10.1128/jvi.52.3.953-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]