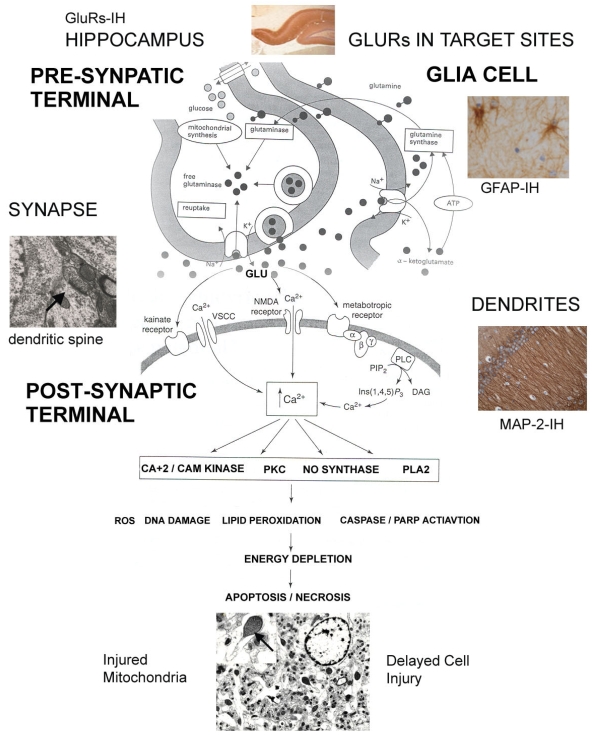
Figure 8.
. Illustration of the key proposed mechanisms of tissue cell injury induced by DOM with photographs of target sites. The diagram shows the interaction between the presynaptic and the postsynaptic terminal. Glutamate receptors in target tissues such as the hippocampus are activated by EEAs such as DOM, which at the same time induces the release of glutamate. The figure shows vesicular release of glutamate into the synaptic space, activation of the post synaptic receptor systems, re-uptake into the pre synaptic terminal, and surrounding glia cells (astrocytes). DOM and glutamate activate the various glutamate receptors present in the postsynaptic membrane, inducing cellular injury through a common injury pathway, a process known as excitotoxicity. This process can be separated into several overlapping components:1)The iGluRs are ion-gated channels selective to Na+, K+ and Ca+2 and any sustained stimulation of these receptors may results in osmotic tissue damage. Hence, neuronal cell swelling reflects the influx of extra cellular Na+, Cl− and water. Focal swellings along the dendrites called varicosities are early structural changes and are viewed as a hallmark of excitotoxic neuronal injury. 2) Activation of iGluRs triggers the influx of Ca+2 from the extra cellular environment to the synaptic cleft. This stage is marked by delayed cell degeneration. The accumulation of Ca+2 is the crucial determinant of injury. This elevation of Ca+2 triggers the activation of several enzymes: calmodulin (CAM), protein kinase (PKC), nitric oxide synthase (NO synthase), phospholipase A2 (PLA2) and reactive oxygen species (ROS). Evidence indicates that the mitochondrion plays a central role in the processes of excitotoxic neuronal cell degeneration with a web of interactions between Ca+2 homeostasis, ATP production, and the generation and detoxification of ROS. 3) GluRs are found localized at the synapse within electron dense structures known as postsynaptic density (PSD), mediating the binding of GluRs to sub membrane proteins such as actin and PDZ containing proteins. These proteins mediate protein-protein interactions. GluRs PDZ-containing proteins bind to numerous signal molecules including nitric oxide synthase, providing a mechanism for clustering GluRs with the corresponding signalling transduction protein. These GluRs associated proteins and excitotoxic signalling result in a free radical cascade and activation of enzymatic processes causing extensive damage of cell structures and ultimate cell death. 4) Astrocytes, as do neurons, express GluRs providing the binding effectors site for DOM and show degenerative structural changes in response to DOM exposure. Failure of astrocytes to remove extra cellular glutamate is one of the key compounding mechanisms implicated in DOM neurotoxicity. (Diagram - modified from Gill and Pulido [131])