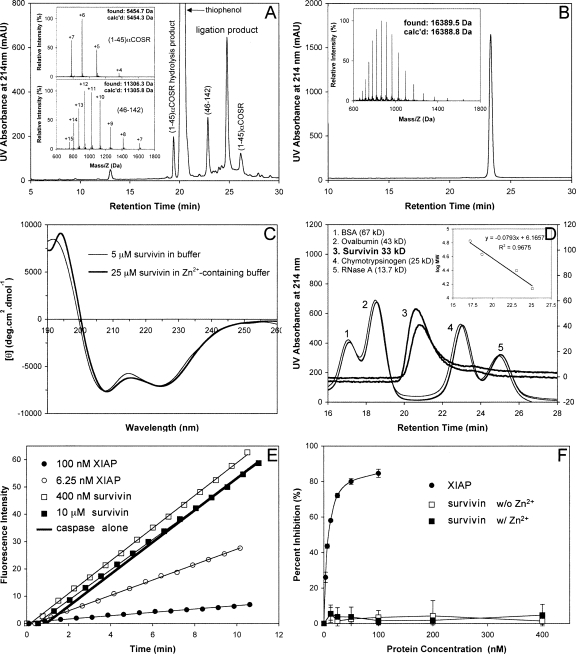
Figure 2.
(A) Ligation of (1–45)αCOSR and (46–142) at 1.5 h. The reaction was monitored by analytical HPLC on a Waters XBridge C18 column (4.6 × 150 mm, 3.5 μM) running a 30-min gradient of 25%–45% acetonitrile containing 0.1% TFA at a flow rate of 1 mL/min. (Insets) Mass spectra of (1–45)αCOSR and (46–142) determined by ESI-MS. (B) Ligated full-length survivin characterized by C18 RP-HPLC and ESI-MS. HPLC conditions: Waters symmetry 300 C18 column (4.6 × 150 mm, 5 μM) running a 30-min gradient of 5%–65% acetonitrile containing 0.1% TFA at a flow rate of 1 mL/min. (C) CD spectra of synthetic survivin at 5 μM in 5 mM phosphate buffer containing 0.1 mM TCEP, pH 7.5 (thin line), and at 25 μM in 5 mM phosphate buffer containing 0.1 mM TCEP and 50 μM Zn2+, pH 7.5 (thick line). (D) Representative size-exclusion chromatograms of synthetic survivin (3) and molecular mass standards (1, 2, 4, 5). Linear regression analysis of the correlation between logarithmic M r and retention time is illustrated in the inset. (E) Representative raw data from the hydrolysis of Ac-DEVD-AMC by caspase-3 in the absence and presence of different concentrations of XIAP and synthetic survivin. (F) Dose-dependent percent inhibition of caspase-3 by XIAP (filled circles), synthetic survivin without Zn2+ (empty squares), and synthetic survivin in the presence of Zn2+ (filled squares). Each curve is the mean of three independent experiments.