Table 1.
Catalyst Screening and Reaction Condition Optimizationsa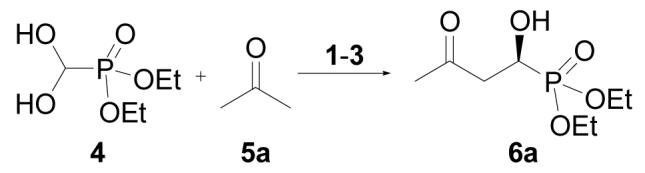
| entry | catalyst | solvent | temp (°C) |
time (h) |
yield (%)b |
ee (%)c |
|---|---|---|---|---|---|---|
| 1 | 1 | acetone | rt | 24 | — | — |
| 2 | 2 | acetone | rt | 24 | 46 | 72 |
| 3 | 3 | acetone | rt | 3 | 93 | 84 |
| 4 | 3 | CH2Cl2 | rt | 10 | 76 | 84 |
| 5 | 3 | DMSO | rt | 10 | 45 | 79 |
| 6 | 3 | DMF | rt | 10 | 55 | 60 |
| 7 | 3 | acetone | 0 | 10 | 95 | 92 |
| 8d | 3 | acetone | 0 | 24 | 95 | 94 |
| 9d | 3 | acetone | -20 | 24 | 62 | 94 |
| 10 | ent-3 | acetone | 0 | 24 | 91 | 92e |
Unless otherwise indicated, all reactions were performed with freshly prepared diethyl formylphosphonate hydrate (4, 0.5 mmol), acetone (0.2 mL), solvent (0.4 mL) and catalyst (10 mol %) at 0 ° C.
Yield of isolated product after column chromatography.
The enantiomeric excess was determined by chiral GC analysis with a Chiraldex GTA column.
With 5 mol % of catalyst loading.
(R)-6a was obtained as the major enantiomer.
