Table 2.
Enantioselective Cross Aldol Reaction of Diethyl Formylphosphonate Hydrate (4) and Various Ketones.a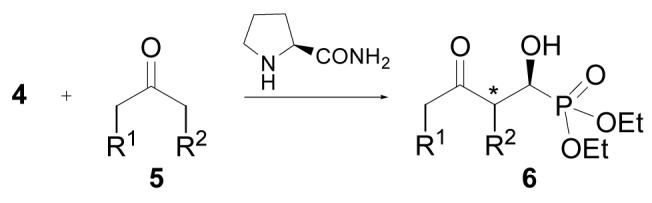
| entry | R1 | R2 | product/ yield (%)b |
drc | ee (%)d |
|---|---|---|---|---|---|
| 1 | H | H | 6a/95 | — | 94e |
| 2 | Me | H | 6b/62f | — | 93e |
| H | Me | 6b’ /20f | 95:5g | 99e | |
| 3 | Et | H | 6c/58f | — | 97e |
| H | Et | 6c’/5f | >95:5g | 99e | |
| 4 | Cl | H | 6d/65 | — | 90e |
| 5 | OH | H | 6e/75 | — | 43h |
| 6 | OMe | H | 6f/77 | — | 85 |
| 7 | AcCH2 | H | 6g/60 | — | 86 |
| 8 | —(CH2)2— | 6h/ 94 | 95:5i | >99 | |
| 9 | —(CH2)3— | 6i/82 | 65:35g | 93(86) | |
| 10j | —(CH2)3— | 6i/90k | 65:35g | 96(95) | |
| 11j | —CH2OCH2— | 6j/85k | 70:30g | 97(99) | |
| 12j | —CH2SCH2— | 6k/88k | 70:30g | 93(97)l | |
Unless otherwise indicated, all reactions were performed with freshly prepared diethyl formylphosphonate hydrate (4, 0.5 mmol), ketone (0.6 mL) and L-prolinamide (5 mol %) at 0 °C; the absolute configuration is tentatively assigned for 6b-k on the basis of the reaction mechanism.
Yield of isolated product after column chromatography.
Determined by 1H NMR.
Unless otherwise noted, enantiomeric excess was determined by HPLC analyses with a Chiralpak AD-H column; values in parentheses are for the minor diastereomer.
The enantiomeric excess was determined by chiral GC analysis with a Chiraldex GTA column.
Yields of individual regioisomer as determined by 1H NMR.
anti/syn ratio as determined by NMR.
With a Chiralcel OJ-H column.
syn/anti ratio as determined by NMR.
The reaction was performed with 4 (0.5 mmol), ketone (0.2 mL), CH2Cl2 (0.5 mL) and L-prolinamide (10mol %) at -20 °C for 24 h.
Combined yield of two inseparable diastereomers.
With a Chiralcel OD-H column.
