Table 10.
Kinetic resolution of epoxides with alcohols using the oligomeric catalyst from Figure 8.
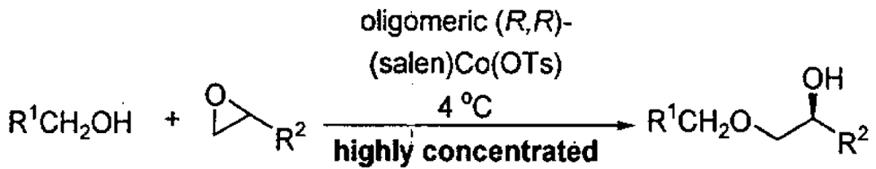 | |||||
|---|---|---|---|---|---|
| entrya | R1 | R2 | Co (mol%)b | yield (%)c,d | ee (%) |
| 1 | Ph | (CH2)3CH3 | 2.0 | 87 | 98 |
| 2 | CH2TMS | (CH2)3CH3 | 0.2 | 97 | 99 |
| 3 | H | (CH2)3CH3 | 0.1 | 96 | 94 |
| 4 | 2-C6H4-Br | (CH2)3CH3 | 0.1 | 99 | 99 |
| 5 | 4-C6H4-OMe | (CH2)3CH3 | 2.0 | 62 | 94 |
| 6 | 2-C6H4-NO2 | (CH2)3CH3 | 0.5 | 98 | 99 |
| 7 | CH=CH2 | (CH2)3CH3 | 0.5 | 87 | 97 |
| 8 | Ph | CH2Cl | 2.0 | 91 | 98 |
| 9 | Ph | CH2O(allyl) | 2.0 | 95 | 98 |
Reactions were carried out with 5M substrates in CH3CN.
Catalyst loading on a per Co bases relative to alcohol.
Isolated yields based on RCH2OH.
Reaction times 3 - 24 h.
