Table 11.
Comparison of the oligomeric catalyst from Figure 8 and the (salen)Co(OTs) in the kinetic resolution of epoxides with phenol derivatives.
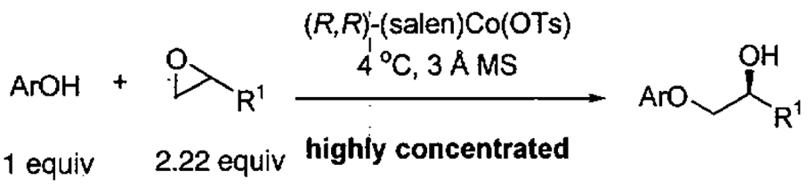 | ||||||
|---|---|---|---|---|---|---|
| entry | Ar | R1 | (R,R)-cat. | Co (mol%)c | yield (%)d,e | ee (%) |
| 1 | Ph | CH2Cl | (salen)Co(OTs)a | 4.0 | 96 | 99 |
| oligomerb | 0.25 | 99 | 99 | |||
| 2 | 2-C6H4-(Oallyl) | CH2Cl | (salen)Co(OTs)a | 4.4 | 48 | 84 |
| oligomerb | 0.25 | 99 | 98 | |||
| 3 | Ph | Ph | (salen)Co(OTs)a | 4.0 | f | n.d. |
| oligomerb | 1.0 | 60 | 97 | |||
| 4 | Ph | c-hexyl | (salen)Co(OTs)a | 8.0 | 89 | 94 |
| oligomerb | 0.5 | 99 | 98 | |||
| 5 | 2-C6H4-Cl | n-Bu | (salen)Co(OTs)a | 4.0 | 80g | 68 |
| oligomerb | 0.8 | 98 | 99 | |||
5M substrates in TBME.
5M substrates in CH3CN.
Catalyst loading on a per Co basis relative to ArOH.
Isolated yields based on ArOH.
Reaction times 4 - 24 h unless otherwise noted.
After 10 days 63% conversion, 2:3 mixture favoring internal attack.
72 h reaction time.
