Table 16.
Asymmetric HDA catalyzed by chiral porphyrin complexes.
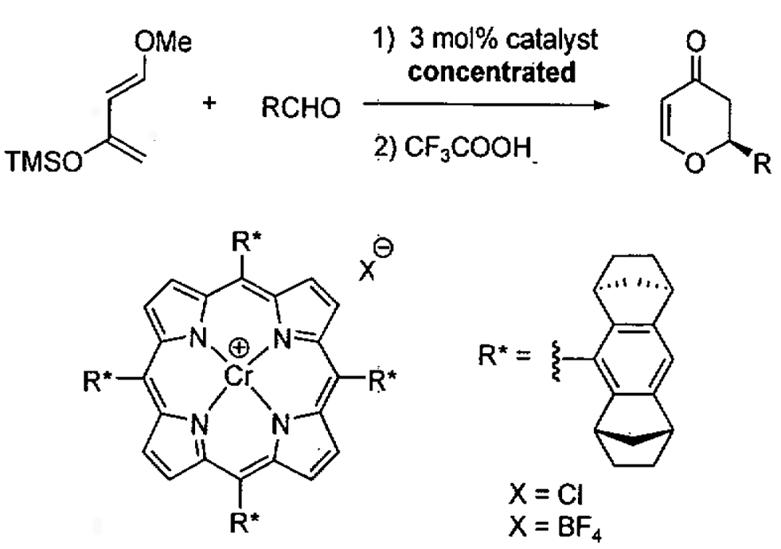 | |||||
|---|---|---|---|---|---|
| entrya | R | catalyst counterion (X−) |
temp (°C) | yield (%) | ee (%) |
| 1 | Ph | Cl− | −18 | 85 | 95 |
| 2 | Ph | BF4− | −18 | 92 | 88 |
| 3 | c-hexyl | Cl− | −18 | 76 | 88 |
| 4 | n-hexyl | Cl− | −18 | 75 | 92 |
| 5 | 2-furyl | Cl− | −18 | 70 | 97 |
| 6 | (E)-CH=CHPh | Cl− | 0 | 55 | 74 |
| 7 | 2-pyridyl | Cl− | −18 | 70 | 78 |
The reactions were performed at 2.0M substrate concentration in MTBE.
