Table 31.
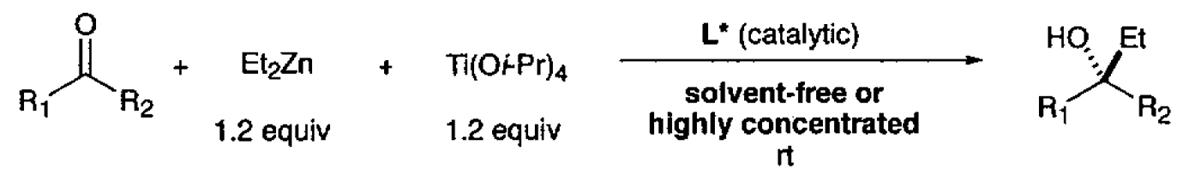
Diethylzinc additions to ketones under solvent-free, highly concentrated, and standard conditions.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| solvent-free or highly concentrated conditions |
standard conditions |
||||||||
| entry | substrates | L* (moi%) | t (h) | y (%) | ee (%) | L*(mol%) | t (h) | y (%) | ee (%) (config.) |
 |
|||||||||
| 1 | X = H | 1 0.5 |
4 21 |
75 78 |
97a 96a |
2 | 29 | 71 | 96 (S) |
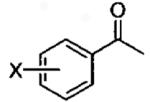
| 2 | X = 3-CF3 | 0.5 | 17 | 77 | 96a | 2 | 14 | 56 | 98 |
| 3 | X = 4-OMe | 1 1 |
12 15 |
50 72 |
81a 89b |
10 | 111 | 85 | 94 |
| 4 | 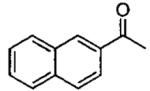 |
0.5 0.5 |
12 24 |
74 76 |
98a 98b |
2 | 27 | 90 | 97 |
| 5 | 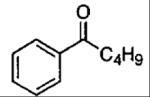 |
1 1 |
24 24 |
78 85 |
80a 80b |
2 | 102 | 79 | 88 (R) |
| 6 | 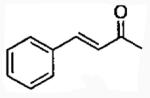 |
1 | 15 | 71 | 90b | 2 | 26 | 80 | 90 |
| 7 | 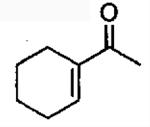 |
1 | 22 | 53 | 93b | 2 | 46 | 56 | 96 |
| 8 | 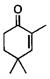 |
1 | 22 | 36 | 96b | 10 | 40 | 76 | 98 |
Solvent-free conditions.
2 Equiv toluene was added to the reaction.
