Table 33.
Comparison of solvent-free, highly concentrated, and standard reaction conditions for the asymmetric addition of functionalized organozinc reagents to ketones.
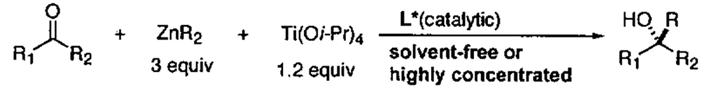 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| entry | substrates | ZnR2 | solvent-free or highly concentrated conditions |
standard conditions |
||||||
| L* (moi%) | t (h) | y (%) | ee (%) | L* (mol%) | t (h) | y (%) | ee (%) | |||
| 1 | 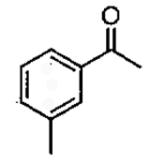 |
Zn((CH2)4OTBS)2 | 1 0.5 0.25 1 |
48 70 82 40 |
68 53 44 68 |
79a 80a 69a 97b |
10 | 72 | 89 | 98 |
| 2 | Zn((CH2)5Br)2 | 1 0.5 1 |
45 50 46 |
66 41 71 |
92a 92a 97b |
10 | 72 | 89 | 96 | |
| 3 | 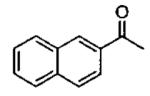 |
Zn((CH2)5Br)2 | 1 0.5 0.25 0.25 1 |
40 76 84 90 38 |
55 47 30 30 72 |
94a 94a 76a 90a,c 97b |
10 | 72 | 55 | 94 |
| 4 | Zn((CH2)3CHMe2)2 | 1 | 18 | 56 | 94b | 10 | 72 | 75 | 90 | |
| 5 | 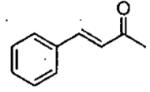 |
Zn((CH2)3CHMe2)2 | 1 | 27 | 63 | 93b | 10 | 72 | 86 | 93 |
| 6 | Zn((CH2)4OTBS)2 | 1 | 21 | 76 | 87b | 10 | 120 | 65 | 90 | |
| 7 | Zn((CH2)5Br)2 | 1 | 36 | 65 | 89b | 10 | 48 | 48 | 90 | |
Solvent-free conditions.
Concentrated reaction conditions (2 equiv toluene was added to the reaction).
0.6 Equiv Ti(Oi-Pr)4 was used.
