Table 40.
Scope of the asymmetric Friedel-Crafts alkylation of aryl ethers with Cu(OTf)2 and ligand A of Figure 37 under solvent-free and highly concentrated reaction conditions.
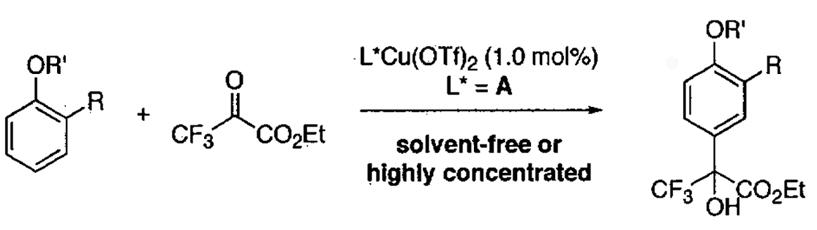 | |||||||
|---|---|---|---|---|---|---|---|
| entry | substrate | temp(°C) | time (h) | product | yield (%) | ee (%) | |
| 1 | 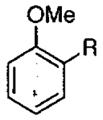 |
R = H | 0 | 10 | 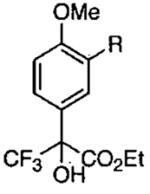 |
90 | 90 |
| 2 | R = Me | −20 | 30 | 80 | 90 | ||
| 3 | R = Ph | −20 | 28 | 55 | 90 | ||
| 4 | 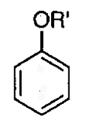 |
R′ = n-Bu | −20 | 40 | 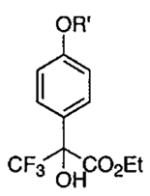 |
88 | 92 |
| 5 | R′ = Ph | 0 | 72 | 62 | 92 | ||
| 6a | R′ = CH2-2-C6H4-Br | 15 | 18 | 96 | 92 | ||
| 7 | R′ = CH2-4-C6H4-Br | 25 | 36 | 85 | 91 | ||
| 8 | R′ = CH2-Ph | 0 | 16 | 98 | 90 | ||
| 9 | R′ = CH2-3-C6H4-Cl | 0 | 28 | 78 | 93 | ||
| 10 | 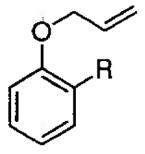 |
R = H | −20 | 30 | 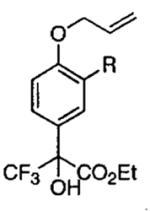 |
62 | 93 |
| 11 | R = Me | −20 | 30 | 75 | 90 | ||
| 12 | R = Ph | −20 | 48 | 70 | 93 | ||
highly concentrated with CH2Cl2.
