Table 49.
Conjugate addition of TMSCN to α,β-unsaturated imides.
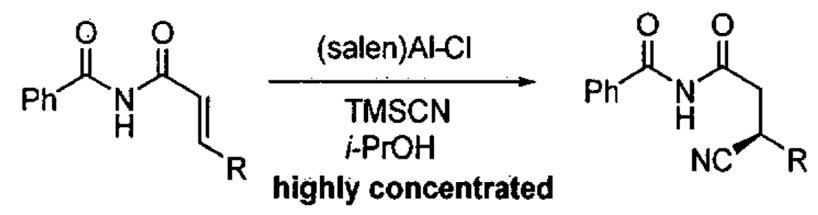 | |||||
|---|---|---|---|---|---|
| entry | R | methoda | time (h) | yield (%) | ee (%) |
| 1 | Me | A | 26 | 92 | 98 |
| 2 | Et | A | 26 | 95 | 97 |
| 3 | n-Pr | A | 26 | 90 | 97 |
| 4 | i-Pr | A | 26 | 91 | 94 |
| 5 | i-Bu | A | 26 | 93 | 96 |
| 6 | (CH2)3CH=CH2 | A | 48 | 96 | 95 |
| 7 | i-Bu | B | 48 | 90 | 97 |
| 8 | CH2OBn | B | 48 | 70 | 87 |
Reactions were carried out on a 0.5 mmol scale. Method A: 10 mol% (S,S)-(salen)Al-Cl, 2.5 equiv of TMSCN, 2.5 equiv 2-propanol, 0.2 mL of toluene, 24 °C. Method B: 15 mol% (S,S)-(salen)Al-Cl, 4 equiv of TMSCN, 4 equiv 2-propanol, 0.4 mL of toluene, 24 °C.
