Table 5.
Kinetic resolution of racemic terminal epoxides with Me3SiN3 catalyzed by (R,R)-(salen)CrN3 under solvent-free conditions.
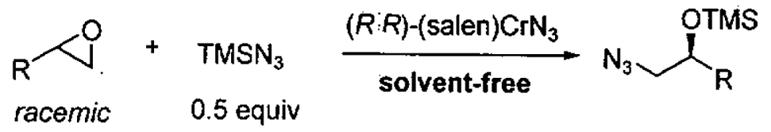 | |||||
|---|---|---|---|---|---|
| entry | R | cat. mol% | yield (%)b | ee (%) | krel |
| 1 | CH3 | 1.0 | 98 | 97 | 230 |
| 2 | CH2CH3 | 2.0 | 83 | 97 | 140 |
| 3 | (CH2)3CH3 | 2.0 | 89 | 97 | 160 |
| 4 | CH2OTBDMS | 3.0 | 96 | 96 | 150 |
| 5 | CH2O(1-naphthyl) | 5.0 | 74 | 93 | 48 |
| 6 | CH2C6H5 | 2.0 | 94 | 93 | 71 |
| 7 | c-C6H11 | 2.0 | 84c | 97 | 140 |
| 8 | (CH2)2CH=CH2 | 2.0 | 94 | 98 | 280 |
| 9 | CH(OEt)2 | 2.0 | 96 | 89 | 44 |
| 10 | CH2CN | 2.0 | 80 | 92 | 45 |
Reactions were run without solvent for 18-50 h at 0-2 °C.
Isolated yield of the azido sily ether based on TMSN3.
Isolated yield of the azido alcohol.
