Table 51.
Solvent-free MBH reaction catalyzed by organocatalysts A – C and DABCO (20 mol% each) from Figure 50.
| entrya | product | cat | yield (%) | ee (%) |
|---|---|---|---|---|
| 1 | 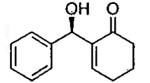 |
A | 65b | 77 |
| 2 | C | quantc | 60 | |
| 3 | 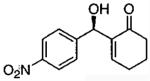 |
A | quantc | 38 |
| 4 | C | 65c | 34 | |
| 5 | 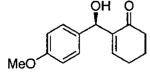 |
A | 35c | 79 |
| 6 | C | 79c | 50 | |
| 7 | 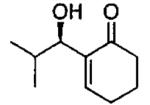 |
A | 62c | 90 |
| 8 | 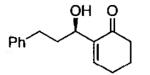 |
A | 28c | 79 |
| 9 | C | 52b | 69 | |
| 10 | 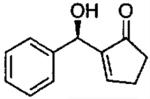 |
C | quantb | rac |
| 11 | 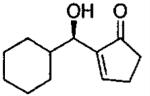 |
C | 22b | 58 |
| 12 | 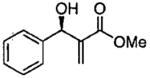 |
C | quantb | rac |
| 13 | 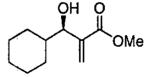 |
B | 47c | 22 |
The reaction was carried out with 1 equiv of aldehyde and 4 equiv of enone or acrylate in the presence of 20 mol% catalyst and DABCO at 10°C for 72 h.
GC yield.
Isolated yield.
