Table 7.
Measured krel values for a series of epoxides under solvent-free and highly concentrated reaction conditions.
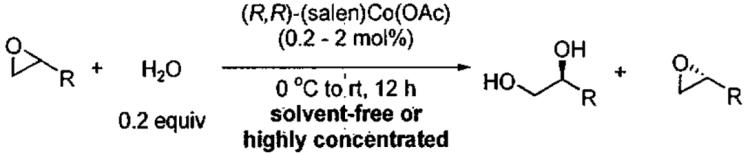 | ||||
|---|---|---|---|---|
| entry | epoxide substituent | conv. (%)c | diol ee (%) | krel |
| Aliphatic Epoxides | ||||
| 1a | CH3 | 19 | 99.5 | 500 |
| 2a | (CH2)3CH3 | 19 | 99.2 | 310 |
| 3b | (CH2)11CH3 | 18 | 99.5 | 490 |
| 4b | (CH2)2CH=CH2 | 20 | 99.4 | 420 |
| 5b | CH2Ph | 20 | 97.4 | 96 |
| 6b | c-C6H11 | 19 | 99.6 | 630 |
| 7b | t-C4H9 | 16 | 97.0 | 79 |
| Halogenated Epoxides | ||||
| 8b | CH2Cl | 20 | 98.7 | 190 |
| 9b | CH2Br | 20 | 96 | 49 |
| 10a | CH2F | 17 | 98 | 120 |
| 11a | CF3 | 18 | 99.6 | 620 |
| Epoxides Bearing Ether and Carbonyl Functionality | ||||
| 12b | CH2OBn | 20 | 97 | 83 |
| 13b | CH2OTBS | 18 | 99 | 250 |
| 14b | CH2OPh | 18 | 98 | 120 |
| 15b | CH2O(1-naphthyl) | 20 | 99 | 250 |
| 16b | CH2CH2OBn | 19 | 97 | 82 |
| 17b | oxiranyld | 20 | 98 | 130 |
| 18b | CH2OCOn-C3H7 | 54 | 99.4 | 68 |
| 19b | CH2CO2Et | 20 | 98 | 130 |
| 20b | CH2NHBoc | 18 | 74 | 7.8 |
| 21b | CO2CH3 | 19 | 98 | 120 |
| 22b | COCH3 | 18 | 97 | 81 |
| 23b | COCH2CH3 | 18 | 96 | 60 |
| Aryl, Vinyl, and Alkynyl Epoxides | ||||
| 24b | C6H5 | 20 | 98 | 130 |
| 25b | 4-ClC6H4 | 18 | 97 | 81 |
| 26b | 3-ClC6H4 | 17 | 98 | 120 |
| 27b | 3-(CH3O)C6H4 | 19 | 98 | 120 |
| 28b | 3-(NO2)C6H4 | 19 | 99 | 280 |
| 29b | 2-ClC6H4 | 18 | 98 | 120 |
| 30b | CH=CH2 | 18 | 98 | 120 |
| 31b | CCTBS | 19 | 99.4 | 420 |
Solvent-free.
Highly concentrated with epoxide : THF = 1 : 1 v/v ratio.
Isolated yield of 1,2-diol.
The substrate was d,l-butadiene diepoxide.
