Table 8.
Comparison of the HKR with (salen)Co(OAc) and (salen)Co(OTs) with various epoxides.
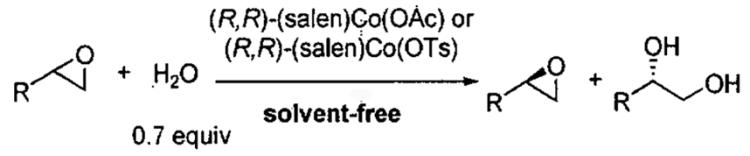 | ||||
|---|---|---|---|---|
| epoxide | catalyst | catalyst (mol%)a |
time (h) |
yield (%)b |
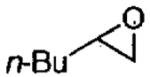 |
(salen)Co(OAc) | 0.5 | 16 | 43 |
| (salen)Co(OTs) | 0.15 | 3 | 44 | |
| (saten)Co(OTs) | 0.05 | 16 | 45 | |
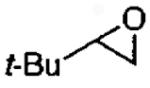 |
(salen)Co(OAc)c | 2.0 | 48 | 40 |
| (salen)Co(OTs)c | 1.2 | 48 | 39 | |
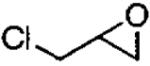 |
(salen)Co(OAc) | 0.5 | 16 | 43 |
| (salen)Co(OTs) | 0.2 | 16 | 42 | |
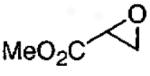 |
(salen)Co(OAc) | 2.0 | 24 | 43 |
| (salen)Co(OTs) | 0.5 | 16 | 42 | |
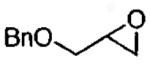 |
(salen)Co(OAc) | 0.5 | 16 | 47 |
| (salen)Co(OTs) | 0.06 | 16 | 45 | |
Catalyst loading based on racemic epoxide.
Isolated yield of >99% ee epoxide based on racemate (theoretical maximum 50%).
Reaction at 0-4 °C.
