Table 9.
Catalyst comparison in the HKR of terminal epoxides with the oligomeric catalyst (Figure 8) and the standard (R,R)-(salen)Co(OAc).
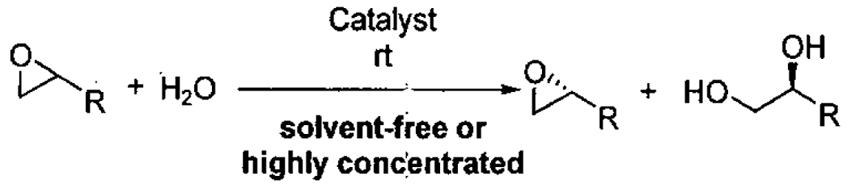 | |||||
|---|---|---|---|---|---|
| entry | isolated product | catalyst | Co (mol%)a | time (h)% | yieldf (% ee) |
| epoxidesg | |||||
| 1b | 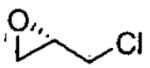 |
monomer oligomer |
0.5 0.01 |
18 11 |
42(99) 45(99) |
| 2c | 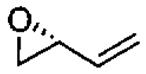 |
monomer oligomer |
0.85 0.05 |
68 24 |
26(99) 36 (99) |
| 3d | 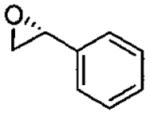 |
monomer oligomer |
0.8 0.08 |
72 24 |
44(99) 37(99) |
| 4e | 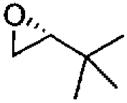 |
monomer oligomer |
2.0 0.1 |
48 24 |
41(99) 41(99) |
| diolsg | |||||
| 5d | 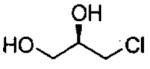 |
monomer oligomer |
2.0 0.01 |
24 1.5 |
50(96) 40(97) |
| 6d | 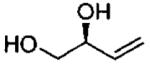 |
monomer oligomer |
0.64 0.03 |
20 4 |
49(94) 46(97) |
| 7d | 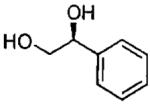 |
monomer oligomer |
0.8 0.08 |
12 4 |
41(98) 43(96) |
| 8d | 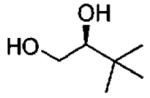 |
monomer oligomer |
2.0 0.05 |
20 18 |
40(95) 49(95) |
Loading on a Co basis relative to racemic epoxide.
Solvent-free.
Highly concentrated reaction using butyronitrile as solvent.
Highly concentrated reaction using 1:1 CH2Cl2: CH3CN as solvent.
Highly concentrated reaction using 1,2-hexanediol as solvent.
Isolated yield based on racemic epoxide.
For reactions in which resolved epoxide was targeted, 0.55-0.60 equiv H2O was used for the resolution. For recovery of diol, 0.45 equiv H2O was employed.
