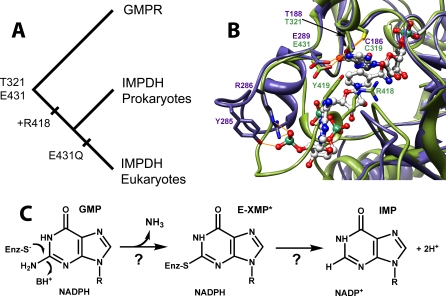
Figure 6. The Evolution of the IMPDH/GMPR Family.
(A) Evolutionary tree for IMPDH and GMPR (midpoint rooting). Substitution Glu431Gln has also occurred several times independently in bacterial IMPDH lineages (see Figure S4 and Text S1).
(B) Active sites of IMPDH and GMPR. The closed conformation as observed in the X-ray crystal structure of the E•MZP complex of T. foetus IMPDH (PDB accession number 1pvn [9]) is shown in olive green, with MZP in charcoal. The E•IMP•NADPH complex of human GMPR2 (PDB accession code 2c6q) is shown in slate blue, with IMP and NADPH in gray. N, O, and P atoms are colored blue, red, and sea green, respectively. Potential hydrogen bonds are depicted in gold. The following residues were omitted for clarity: IMPDH, 57–68, 324–328, and 389–393; GMPR, 57–63, 191–195, and 250–256. This figure was rendered with Chimera [53].
(C) The GMPR reaction.