Abstract
The nucleus of the solitary tract (NST) and the parabrachial nuclei (PbN) are the first and second central relays for the taste pathway, respectively. Taste neurons in the NST project to the PbN, which further transmits taste information to the rostral taste centers. Nevertheless, details of the neural connections among the brain stem gustatory nuclei are obscure. Here, we investigated these relationships in the hamster brain stem. Three electrode assemblies were used to record the activity of taste neurons extracellularly and then to electrically stimulate these same areas in the order: left PbN, right PbN, and right NST. A fourth electrode, a glass micropipette, was used to record from gustatory cells in the left NST. Results showed extensive bilateral communication between brain stem nuclei at the same level: 1) 10% of 96 NST neurons projected to the contralateral NST and 58% received synaptic input from the contralateral NST; and 2) 12% of 43 PbN neurons projected to the contralateral PbN and 21% received synaptic input from the contralateral PbN. Results also showed extensive communication between levels: 1) as expected, the majority of 119 NST neurons, 82%, projected to the ipsilateral PbN, but 85% of the 20 NST neurons tested received synaptic input from the ipsilateral PbN, as did 59% of 22 NST neurons that did not project to the PbN; and 2) although few, 3%, of 119 NST cells projected to the contralateral PbN and 38% received synaptic input from the contralateral PbN. These results demonstrated that taste neurons in the NST not only project to, but also receive descending input from the bilateral PbN and that gustatory neurons in the NST and PbN also communicate with the corresponding nucleus on the contralateral side.
INTRODUCTION
The nucleus of the solitary tract (NST) in the medulla and the parabrachial nuclei (PbN) in the pons are the first and second taste relays in the rodent CNS, respectively (Lundy Jr and Norgren 2004b; Travers 1988). The NST and PbN are divided into anatomically distinct subnuclei (Davis 1991; Davis and Jang 1988; Halsell and Frank 1991; Renehan et al. 1994, 1996; Whitehead 1988). Taste-responsive neurons are found mostly in the rostral central and rostral lateral subdivisions in the NST (Cho et al. 2002b; Li et al. 2002; McPheeters et al. 1990; Monroe and Di Lorenzo 1995). The gustatory PbN cells are primarily located in the medial subdivisions (Halsell and Frank 1992; Norgren and Pfaffmann 1975; Ogawa et al. 1984a; Travers and Smith 1984; Van Buskirk and Smith 1981). The medial PbN is where anterogradely labeled axons were observed following tracer injection into the rostral NST and retrogradely labeled cell bodies were located after the injection of the tracer into the parvicellular division of the ventroposteromedial nucleus of the thalamus (VPMpc) (Halsell 1992; Norgren 1976; Voshart and van der Kooy 1981; Whitehead et al. 2000; Williams et al. 1996).
The extranuclear projections of the rostral NST neurons reach to the PbN or terminate within the medulla including the contralateral NST (Halsell et al. 1996; Norgren 1978; Norgren and Leonard 1973; Travers 1988; Travers and Hu 2000; Whitehead 1990; Whitehead et al. 2000; Williams et al. 1996). PbN-projecting NST neurons are mostly located in the central and lateral subnuclei, whereas the cells that send axons to the reticular formation or caudal NST are largely found in the ventral subdivision of the rostral NST (Beckman and Whitehead 1991; Halsell et al. 1996; Streefland and Jansen 1999; Travers and Hu 2000). Although the pontine and intramedullary projections arise largely from separate populations, following separate retrograde tracer injections into the PbN and the caudal NST some rostral NST neurons were double labeled (Halsell et al. 1996). However, the possibility of rostral NST neurons projecting both to the PbN and to the contralateral NST has not been investigated.
The projection of NST taste neurons to the ipsilateral, but not the contralateral, PbN was investigated electrophysiologically in the rat and hamster (Cho et al. 2002a; Monroe and Di Lorenzo 1995; Ogawa and Kaisaku 1982; Ogawa et al. 1984b), although a contralateral projection has been demonstrated anatomically in the rat and hamster (Whitehead et al. 2000; Williams et al. 1996). In the hamster, some NST taste cells that do not project rostrally do respond orthodromically to ipsilateral PbN stimulation (Cho et al. 2002a). Whether PbN-projecting NST taste neurons receive efferent projections from either the ipsi- or contralateral PbN remains to be determined. The internuclear projections between the rostral NST (Whitehead et al. 2000) or PbN (Moga et al. 1990) were reported neuroanatomically. However, whether these neural connections are gustatory in nature remains undetermined. The purpose of the present study was to investigate all the possible interconnections between the bilateral brain stem gustatory nuclei in the hamster.
METHODS
Animal surgery
The experimental procedures used here were approved by the Institutional Animal Care and Use Committee of Southern Illinois University at Carbondale. Young adult male Syrian golden hamsters (Mesocricetus auratus), weighing between 132 and 176 g (n = 25) were deeply anesthetized with urethane (1.7 g/kg, administered intraperitoneally). Additional anesthetic (10% of original dose) was given as needed during the course of each experiment to maintain anesthesia. Each animal was tracheotomized and mounted in a stereotaxic instrument (Narishige SR-6N) using a nontraumatic head holder with the snout angled downward 27° from horizontal to straighten the brain stem and minimize brain movement associated with breathing (Erickson 1966). A sagittal skin incision was made along the midline overlying the posterior skull and a portion of the occipital bone just dorsal to the foramen magnum was removed to reveal the cerebellum. The dura covering the cerebellum was excised and the posterior portion of the cerebellum was aspirated for 5–6 mm anterior to the obex, allowing direct access to the PbN and NST. Body temperature was monitored and maintained at 37 ± 1°C with an electric heating pad.
Single-unit recording and electrical stimulation
Two types of electrodes were used in our experiments. To record from and stimulate the PbN bilaterally and the NST unilaterally, three concentric recording/stimulating electrode assemblies were used. Each concentric recording/stimulating electrode was constructed by inserting an Epoxylite-insulated 33-gauge stainless steel tube into a 27-gauge stainless steel tube; the two were insulated from each other and cemented together with Epoxylite 6001 (Epoxylite, Irvine, CA). The inner tubing protruded 500 μm from the outer barrel and was exposed at its tip for 200 μm. The tip area of the outer tubing was exposed concentrically for 150 μm. A tungsten microelectrode (tip diameter = 1–2 μm; shank diameter = 75 μm; resistance = 10 MΩ, FHC, Bowdoinham, ME) was inserted through the inner barrel of the stimulating electrode; its tip protruded 1.0 mm from the tip of the inner barrel. A fourth electrode, a single-barrel glass micropipette (tip diameter = 1–2 μm; resistance = 5–7 MΩ) filled with 2% (wt/vol) solution of Chicago Sky Blue dye (Sigma, St. Louis, MO) in 0.5 M sodium acetate was used for extracellular single-unit recording of action potentials from the remaining (ipsilateral) NST. Extracellular action potentials were displayed on oscilloscopes and monitored with an audio monitor (Grass AM8 audio monitor, Grass Instruments, Quincy, MA). Action potentials were amplified (NeuroLog, Digitimer, Hertfordshire, UK), discriminated (Bak DDIS-1, Bak Electronics, Germantown, MD), and then analyzed with Spike2 software (Cambridge Electronic Design, Cambridge, UK).
A combined recording/stimulating electrode was initially positioned about 4.0 mm rostral to the obex and 1.4 mm lateral to the midline to search for the PbN taste-responsive region in one side. While recording neural activity, the electrode assembly was lowered slowly into the pons until a single taste neuron was identified. Taste-responsive neurons were defined as those that fired action potentials in response to chemical stimulation of the anterior tongue. Taste neurons that were recorded by the first electrode assembly in the PbN were not included in the analysis. At this point, the assembly was lowered for an additional 1.0 mm to position the stimulation tip at the depth where the taste neuron had been isolated. The electrode was then fixed to the adjacent skull with dental cement.
This process was repeated with a second recording/stimulating electrode assembly in the contralateral PbN. Once a PbN taste neuron was isolated, but prior to fixing the second assemblage, its responsiveness to stimulation of the opposite PbN was assessed (0.5-ms duration, ≤0.1 mA at 1/3 Hz, Grass 88; Grass Instruments). After testing several taste neurons in this manner, the second electrode assembly was advanced an additional 1.0 mm and fixed to the skull.
A third recording/stimulating electrode was placed in the rostral NST beginning 2.1 mm rostral to the obex and 1.3 mm lateral to the midline. After a taste-responsive NST neuron was identified with this assemblage, its responsiveness to independent stimulation of each side of the PbN was tested. Following several such recordings, this third electrode assembly was also lowered an additional 1.0 mm and fixed to the adjacent skull. Finally, a single-barrel glass micropipette was used to record taste neurons from the ipsilateral NST. Once a taste neuron was isolated and its taste profile was established, its responsiveness to each of the three stimulating electrodes was tested.
Classification of taste neurons by their responsiveness to electrical stimulation
Each PbN and NST taste neuron was examined for antidromic and orthodromic activation in response to electrical stimulation of the other nuclei as described earlier. The criteria for antidromic activation were constant latency and the ability to follow a stimulus pulse pair at >100 Hz. A collision test was performed between a spontaneously generated action potential and a stimulus-evoked action potential. Since the pulse pair (>100 Hz) delivered to the axon of the recorded cell was triggered by a spontaneously generated action potential that invades the axon in an orthodromic direction from the soma, the first stimulus-evoked antidromic action potential of the pulse pair collides with the spontaneously generated action potential and is cancelled, whereas the second stimulus-evoked antidromic action potential reaches the soma from the stimulating site of the axon. Antidromic electrical activation defines a recorded neuron that projects an axon to the electrical stimulation site. Antidromically activated cells were classified as projection neurons. Each neuron in both the PbN and NST was tested first to determine whether it could be activated antidromically from each stimulating site. Orthodromic responsiveness was also examined in a subset of cells that were antidromically activated and all neurons that were not antidromically activated. Orthodromic activation defines neurons that project from the electrically activated site to the recorded neuron past a synapse. A peristimulus time histogram (PSTH, 1-ms bin width) was created from the acquired data on each PbN or NST cell; action potentials associated with single-pulse stimulation were accumulated over a 1-s period for 50–200 stimulus pulses (0.5 ms, 0.1 mA, 1/3 Hz) delivered to each of the stimulating electrodes. If a cell responded neither antidromically nor orthodromically, it was classified as a nonresponsive neuron.
Taste stimulation and classification of taste neurons by their taste profile
Taste stimuli presented to the anterior tongue were 32 mM sucrose, 32 mM sodium chloride (NaCl), 32 mM quinine hydrochloride (QHCl), and 3.2 mM citric acid as used in our previous experiments (Cho et al. 2002a, 2003; Li and Cho 2006). The taste solutions were delivered by a gravity-flow system composed of a computer-controlled two-way solenoid-operated valve connected by tubing to a distilled water rinse reservoir and a stimulus funnel. The stimulation sequence was a continuous flow initiated by the delivery of distilled water for 10 s, followed by 10 s of stimulus, followed by 10 s of distilled water rinse. The flow rate was 2.0 ml/s. After each tastant, the tongue was rinsed with distilled water (>50 ml) and individual stimulations were separated by ≥2.0 min to avoid adaptation effects (Smith and Bealer 1976). Each gustatory neuron was classified as belonging to one of the four best-stimulus categories on the basis of its strongest response to one of the four taste stimuli. These categories were NaCl-best (N-best), sucrose-best (S-best), citric acid-best (C-best), and QHCl-best (Q-best).
Data analysis
The responses of each cell to taste stimulation of the tongue were accumulated over three consecutive sequences composed of 10 s of distilled water, 10 s of stimulus flow, and 10 s of water rinse. The baseline activity of the cell was defined as the mean firing during the 5 s of prerinse water application immediately prior to the taste delivery. The net taste response was calculated as the mean number of action potentials (impulses/s) during the first 5 s of chemical stimulation minus the number of spikes during 5 s of distilled water before the taste delivery. Responses are reported as means ± SE. A taste response was defined as effective if it was ≥2SD above the baseline discharge, which was calculated from the firing rates during the 5-s periods of prerinse water before each of the four taste stimuli. The entropy of each neuron, which is a measure of its breadth of responsiveness across the four taste stimuli, was calculated as described previously (Li and Cho 2006; Smith and Travers 1979).
For orthodromic responses of PbN or NST cells to electrical stimulation of the PbN or NST, an individual PSTH was analyzed to determine excitatory or inhibitory epochs as previously described (Cho et al. 2003). The 200 ms preceding stimulation was identified as the baseline period. The mean and SD of the number of spikes/1-ms bin during this baseline period were determined. An excitatory response was defined as an epoch of at least five consecutive 1-ms bins with a mean value of 2SD above the baseline mean, which defines a mean response with a probability of <0.05. The onset latency of the excitatory response was defined as the time at which the firing rate became at least twice the average baseline spontaneous rate. Inhibitory responses were defined as ≥40 consecutive bins in which the mean value was ≥50% less than that during the baseline. The connectivity between each stimulating and recording site was defined as follows. If a recorded cell projected to the stimulating site, it was specified as recording site-to-stimulating site (e.g., NST-to-iPbN; i for ipsilateral) or, if it projected from the stimulating site via synapse, it was defined as stimulating site-to-recording site via synapse (e.g., cPbN-to-NST via synapse; c for contralateral). In addition, the recording site is indicated simply as PbN or NST, whereas i or c precedes PbN or NST to indicate the stimulating site and its relationship to the recording site.
The number of neurons (percentage) in each category was compared using a chi-square (χ2) analysis. Differences in spontaneous firing rates between the PbN and NST units were compared using a t-test. Univariate ANOVA was used to compare differences between PbN and NST neurons in mean firing rates to a single taste stimulus, across taste stimuli, and in entropies among best stimuli. Pearson's correlation coefficient was used to compare pairs of latencies between cPbN-to-NST via synapse and cNST-to-NST via synapse of a subset of NST cells using SPSS software (SPSS, Chicago, IL).
Histology
At the end of each experiment, the last recording site of the day in the NST was marked by passing a 10-μA cathodal current through the recording electrode for 10 min (5 s on–off) to deposit a spot of Chicago Sky Blue dye. The stimulating sites on the contralateral NST and bilateral PbN were also marked by passing 10-μA anodal current through the inner tubing of the recording/stimulating electrode assembly for 20–30 s to deposit a spot of iron. The hamster was then given a lethal overdose of urethane and perfused through the heart with 200 ml of 4% formalin containing 3% potassium ferrocyanide and 3% ferricyanide. The brains were removed, postfixed, frozen sectioned (40 μm) in the coronal plane, and stained with neutral red. The recording and stimulating sites were located microscopically and plotted on the basis of standard atlas sections of the hamster (Morin and Wood 2001).
RESULTS
Histology
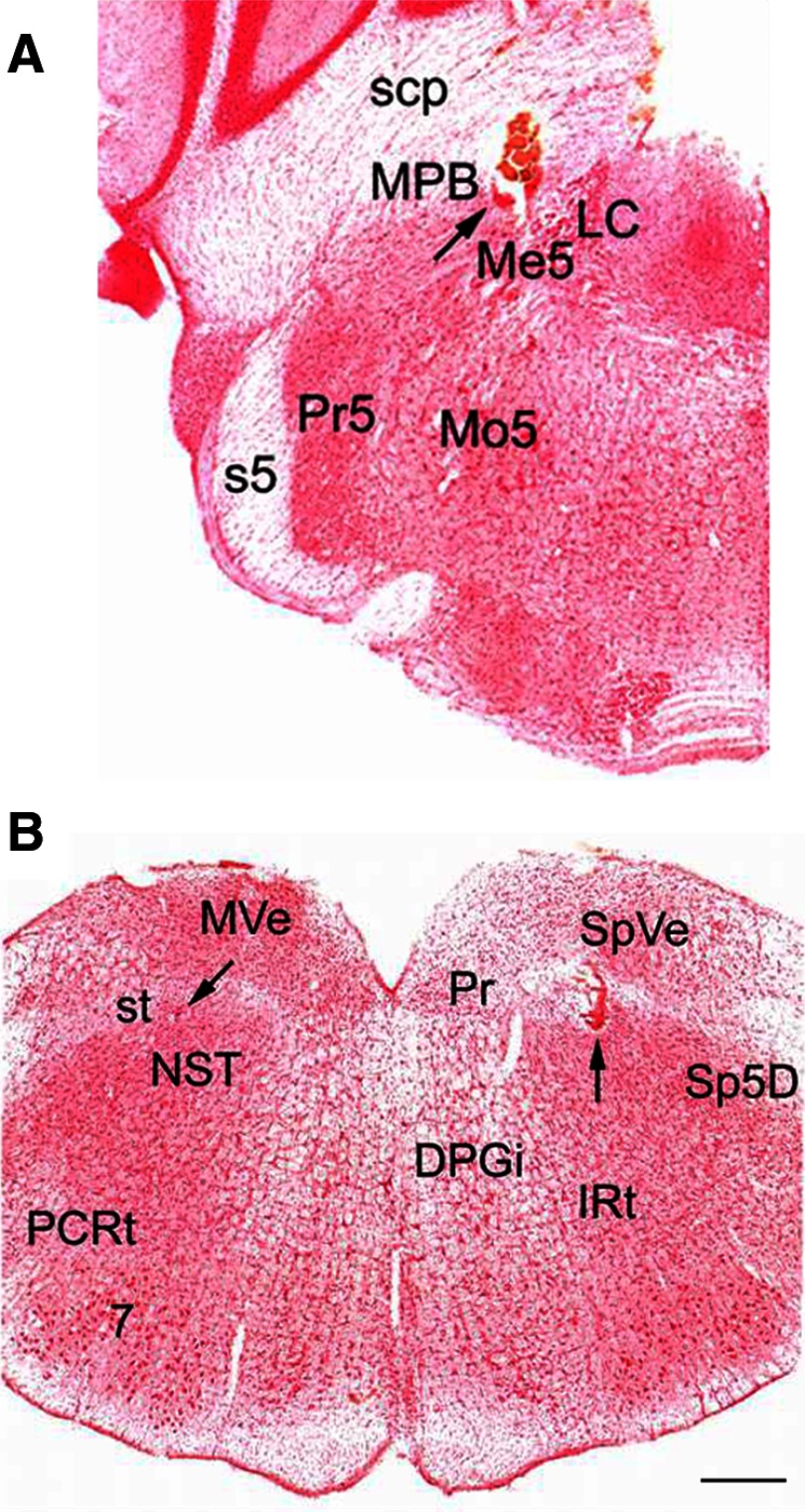
In all, 43 PbN and 119 NST taste-responsive neurons were recorded from 25 male hamsters. The last recording site of the day and the three stimulating sites in each animal were marked and identified histologically (Fig. 1). An iron deposit indicating a PbN stimulating site on the coronal section through the hamster pons, along with an electrode penetration track, is located in the middle of medial PbN (MPB), medial to the superior cerebellar peduncle (scp) at the level where the locus ceruleus (LC) is most evident (Fig. 1A). A recording site in the NST, marked with Chicago Sky Blue dye, is shown on the left (Fig. 1B) medial to the solitary tract An NST stimulating site indicated by an iron deposit, along with an electrode penetration track, is shown on the right side of the same section (Fig. 1B).
FIG. 1.
Photomicrographs of stimulating and recording sites in the hamster brain stem. A: coronal section through the pons showing the position of the stimulating electrode (arrow). Iron deposit and tissue damage indicate a placement within the MPB. B: coronal section through the medulla, showing a recording site, marked with Chicago Blue dye (arrow, left NST) and a stimulating site, marked with iron deposit (arrow, right NST) within the NST. Both photomicrographs were obtained from the sections of the same animal. Abbreviations: 7, facial nucleus; DPGi, dorsal paragigantocellular nucleus; IRt, intermediate reticular nucleus; LC, locus ceruleus; Me5, mesencephalic trigeminal nucleus; Mo5, motor trigeminal nucleus; MPB, medial parabrachial nucleus; MVe, medial vestibular nucleus; NST, nucleus of the solitary tract; PCRt, parvicellular reticular nucleus; Pr, prepositus nucleus; Pr5, principal sensory trigeminal nucleus; s5, sensory root of the trigeminal nerve; scp, superior cerebellar peduncle; Sp5D, spinal trigeminal nucleus, dorsal, part; SpVe, spinal vestibular nucleus; st, solitary tract. Calibration bar = 500 μm.
All the recording and stimulating sites from 25 hamsters were examined and reconstructed using standard atlas sections (figures are not shown). In all the hamsters, the tips of the PbN stimulating electrodes were confined to the PbN on either side, specifically to the MPB, medial to the scp and lateral to the mesencephalic trigeminal nucleus and the LC. The anterior–posterior distribution of the PbN stimulating electrodes extended rostrally from the level at which LC is first apparent and caudally to the appearance of the accessory trigeminal nucleus. In the medulla, the recording marks were confined to the rostral NST, mostly in the rostral central or rostral lateral subdivision at the level of the caudal border of the dorsal cochlear nucleus. This is the area of the NST that receives its predominant gustatory input from the VIIth nerve (Whitehead and Frank 1983). The distribution of the tips of the recording electrodes was similar to that of our previous recordings (Cho et al. 2002a,b, 2003).
Taste responses of PbN versus NST neurons
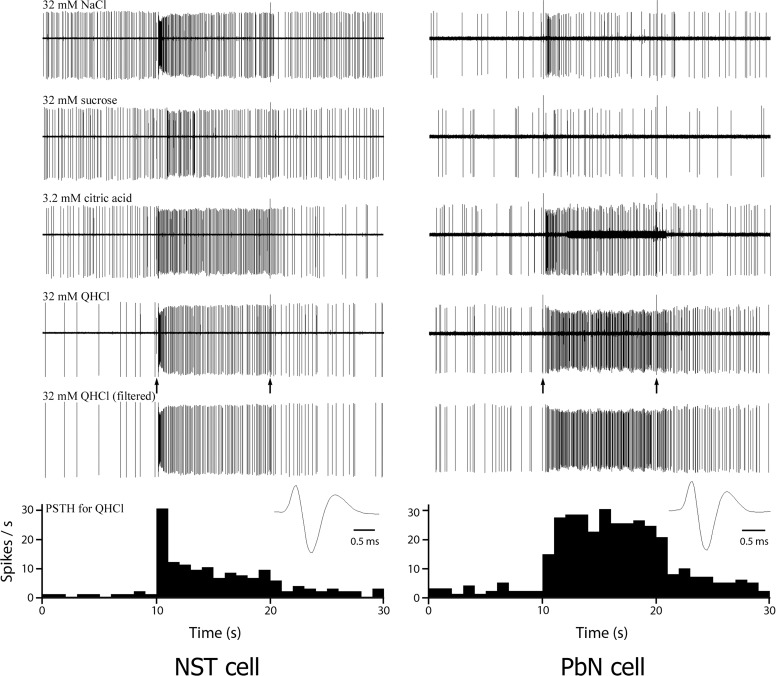
Individual recordings of 43 and 119 gustatory neurons were made in the PbN and the NST, respectively, and each of the 162 cells was tested for its responsiveness to the four basic taste stimuli. Of the 119 cells recorded in the NST, 11 neurons responded to all four taste stimuli, whereas none of the PbN neurons responded to all four stimuli. Q-best (n = 15) and N-best cells (n = 13) were the most frequently recorded in the PbN and N-best (n = 35) and Q-best (n = 34) neurons were the most common in the NST. Recordings from ten of the S-best and five of the C-best cells were obtained in the PbN. Twenty-five neurons from each of the S-best and the C-best types were recorded in the NST. Gustatory responses from representative Q-best neurons from the NST and PbN are shown in Fig. 2. The NST cell in the figure (left) responded to all four taste stimuli, whereas the PbN neuron (right) responded to QHCl, citric acid, and NaCl. The numbers of PbN and NST neurons categorized by their best stimulus and number of effective taste stimuli are summarized in Table 1.
FIG. 2.
Single-unit recordings from an NST (left) and a parabrachial nucleus (PbN) cell (right) in response to taste stimulation of the anterior tongue. The first 4 traces in each panel show 30-s raw recording response to NaCl, sucrose, citric acid, and QHCl stimulation, respectively. Artifacts (at 10 and 20 s) indicate opening (10 s) and closing (20 s) of the solenoids that control the delivery of the stimulus, respectively. Both neurons of the NST and PbN responded best to QHCl and the responses to QHCl of both neurons are replotted with the background activity and stimulus artifacts filtered out in each panel. The peristimulus time histograms (PSTHs) showing the impulse frequencies in response to QHCl, derived from the last recording traced in each panel (QHCl filtered), are shown at the bottom of the figure. The wave forms of the action potential of both units are shown below the last traces.
TABLE 1.
Classification of PbN and NST taste neurons by means of their best stimulus and number of effective taste stimuli
| Best Stimulus | Number of Effective Stimuli | Number of Neurons |
||
|---|---|---|---|---|
| PbN (n = 43) | NST (n = 119) | |||
| N-best | 1 | 7 | 18 | |
| 2 | 5 | 11 | ||
| 3 | 1 | 5 | ||
| 4 | 0 | 1 | ||
| Subtotal: 13 | Subtotal: 35 | |||
| S-best | 1 | 6 | 8 | |
| 2 | 4 | 10 | ||
| 3 | 0 | 3 | ||
| 4 | 0 | 4 | ||
| Subtotal: 10 | Subtotal: 25 | |||
| C-best | 1 | 3 | 10 | |
| 2 | 1 | 8 | ||
| 3 | 1 | 5 | ||
| 4 | 0 | 2 | ||
| Subtotal: 5 | Subtotal: 25 | |||
| Q-best | 1 | 6 | 12 | |
| 2 | 6 | 9 | ||
| 3 | 3 | 9 | ||
| 4 | 0 | 4 | ||
| Subtotal: 15 | Subtotal: 34 | |||
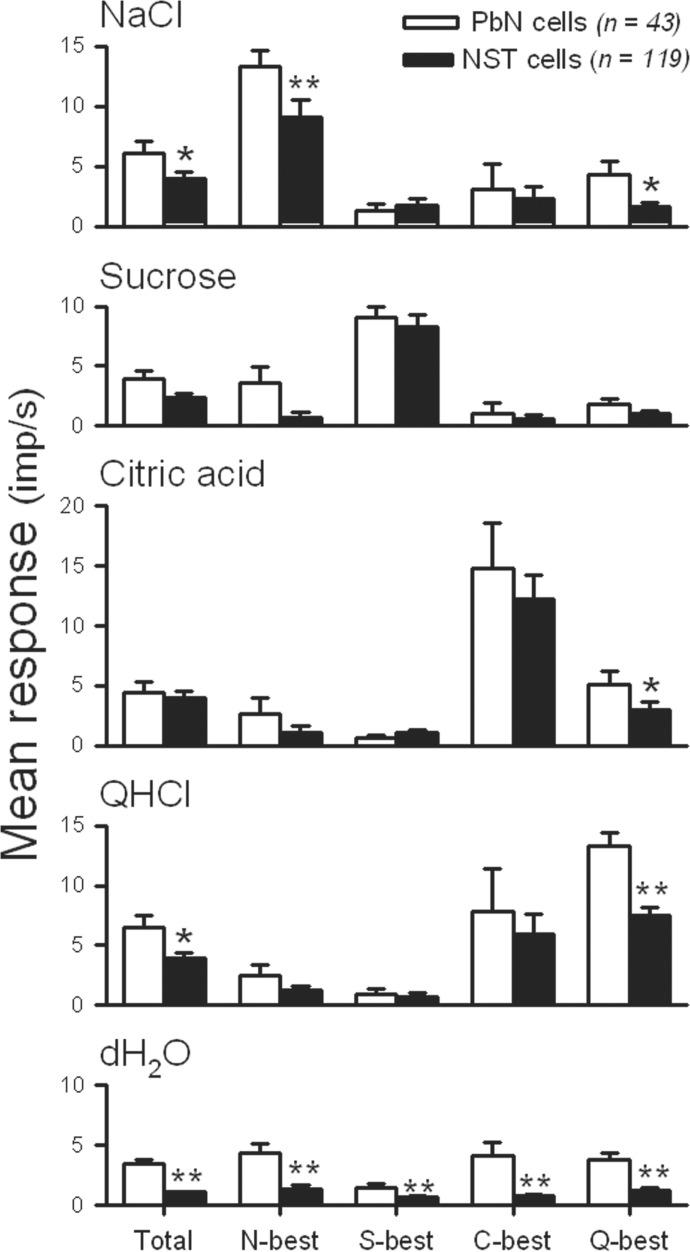
To examine the gustatory responses were different between PbN and NST neurons, and among four taste stimuli, an ANOVA analysis of firing rates in response to each of four taste stimuli of the PbN and NST neurons was computed. The result demonstrated that the overall taste response of PbN neurons was greater than that of NST neurons [F(1,640) = 10.916, P < 0.005]. Responses to taste stimulation were also significantly different among four taste stimuli [F(3,640) = 3.250, P < 0.05], but there was no significant interaction between nuclei and stimuli [F(3,640) = 0.777, P = 0.507]. A comparison by stimulus, however, demonstrated that the response to NaCl (P < 0.05) and QHCl (P < 0.05) in the PbN was significantly greater than the corresponding response in the NST. The mean firing rate in response to each taste stimulus and the mean baseline firing rate of all N-best, S-best, C-best, and Q-best PbN and NST neurons are depicted in Fig. 3. The baseline discharge rate (response to distilled water) ranged from 0.2 to 8.8 impulses/s for PbN cells and 0 to 6.1 impulses/s for NST cells. The baseline firing rates of the 43 PbN taste neurons (mean = 3.45 ± 0.37, impulses/s) was significantly greater than those of the 119 NST taste cells (mean = 1.07 ± 0.11 impulses/s, t = 8.318, df = 160, P < 0.001). These analyses suggest that spontaneous firing rates and gustatory responses of PbN cells are generally greater than those of NST neurons.
FIG. 3.
Comparison of the mean firing rate (±SE, impulses/s) of taste neurons as a function of best-stimulus category (columns) in response to the 4 taste stimuli, and distilled water (rows) in the PbN (open bars) and NST (filled bars). Total, N-best, S-best, C-best, Q-best, and dH2O correspond to the total sample, NaCl-best, sucrose-best, citric acid-best, QHCl-best, and distilled water, respectively. *P < 0.05; **P < 0.01.
The entropy for each taste neuron was obtained from the excitatory components of the responses. The mean entropy of the PbN neurons (0.59 ± 0.04) was not significantly different from that of the NST cells [0.55 ± 0.02: F(1,154) = 0.816, P = 0.368]. In comparison, the difference among the best-stimulus groups was significant [F(3,154) = 3.910, P < 0.05] without significant interaction between the nuclei and best-stimulus groups [F(3,154) = 0.017, P = 0.829]. In both the NST and PbN, S-best cells were most narrowly tuned (0.46 ± 0.08 for PbN S-best and 0.49 ± 0.05 for NST S-best), whereas Q-best cells were most broadly tuned (0.68 ± 0.06 for PbN Q-best and 0.65 ± 0.04 for NST Q-best). The comparison of entropies indicates that sensitivity of the third-order taste cells in the PbN is not different from those of the second-order neurons in the NST.
Effect of stimulation of the contralateral PbN on PbN taste neurons
Forty-three taste-responsive PbN neurons were examined for their responsiveness to electrical stimulation of the contralateral PbN. Of those, 14 (32.6%) neurons were activated: 5 (11.6%) projected to the contralateral PbN and 9 (20.9%) received projection by synapse from the contralateral PbN. More PbN neurons received input via synapse from the contralateral PbN than projected to the contralateral side (χ2 = 23.07, df = 2, P < 0.001). The remaining 29 (67.4%) gustatory PbN neurons did not respond to electrical stimulation of the contralateral PbN. Table 2 summarizes the number of responsive cells for each projection type and the best-stimulus categories. None of the C-best PbN neurons responded to electrical stimulation of the contralateral PbN, whereas more than half of the Q-best cells did.
TABLE 2.
Classification of PbN and NST taste neurons as a function of projection type and best stimulus
| Recording Site Stimulation Site |
PbN |
NST |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contralateral PbN |
Ipsilateral PbN |
Contralateral PbN |
Contralateral NST |
|||||||||||
| Projection Type | From | To | From | To | From | To | From | To | ||||||
| N-best | 1 | 1 | 30 | 3 | 2 | 7 | 3 | 11 | ||||||
| S-best | 2 | 1 | 24 | 0 | 2 | 7 | 1 | 11 | ||||||
| C-best | 0 | 0 | 18 | 5 | 0 | 11 | 3 | 14 | ||||||
| Q-best | 2 | 7 | 25 | 5 | 0 | 20 | 3 | 20 | ||||||
| Total | 5 | 9 | 97 | 13 | 4 | 45 | 10 | 56 | ||||||
Only responsive cells are included in the table. “Projection Type” indicates that a projection is originated “From” or directed “To” a recorded neuron.
Orthodromic responses were all excitatory and the duration of excitatory epoch ranged from 12 to 41 ms. The response latencies of cPbN-to-PbN via synapse (14–47 ms) were longer than the onset latencies of PbN-to-cPbN (3.8–11.2 ms). The thresholds for antidromic activation ranged from 48 to 83 μA. The mean latencies, antidromic thresholds, and durations of the excitatory responses are described in Table 3. It was reported that anesthetic agents, including urethane, increased latencies and decreased the amplitude of rat cortical neuronal activity in response to electrical stimulation to the forepaw (Angel and Gratton 1982). It was also indicated that urethane reduced excitatory neurotransmission in the rat hippocampus (Shirasaka and Wasterlain 1995). Therefore the actual latencies in unanesthetized animals may actually be shorter than we report here.
TABLE 3.
Summary of mean latency, threshold, and response duration of taste neurons as a function of the neural connection
| Projection Typea | Mean Latency, ms | Mean Threshold, μA | Number of Cells | Projection Typeb | Mean Latency, ms | Mean Response Duration, ms | Number of Cells |
|---|---|---|---|---|---|---|---|
| PbN-to-cPbN | 7.5 ± 1.3 | 67.6 ± 6.8 | 5 | cPbN-to-PbN | 29.9 ± 3.8 | 30.2 ± 3.2 | 9 |
| NST-to-iPbN | 4.7 ± 0.4 | 55.1 ± 2.5 | 97 | iPbN-to-NST | 11.6 ± 1.2 | 18.2 ± 2.6 | 13 |
| (11.3 ± 0.7*) | (16.6 ± 1.4*) | (30*) | |||||
| NST-to-cPbN | 9.4 ± 3.4 | 70.3 ± 15.0 | 4 | cPbN-to-NST | 36.7 ± 1.5 | 31.9 ± 1.5 | 45 |
| NST-to-cNST | 15.9 ± 2.2 | 75.4 ± 6.3 | 10 | cNST-to-NST | 22.6 ± 0.7 | 26.6 ± 1.3 | 56 |
Values are means ± SE. Projection type:
from the recorded neuron to the stimulating site;
from the stimulating site to the recorded neuron via synapse(s).
indicates means of 30 NST taste neurons, which included the 17 ipsilateral PbN-projecting cells (see Effect of stimulation of the bilateral PbN on NST taste neurons in results).
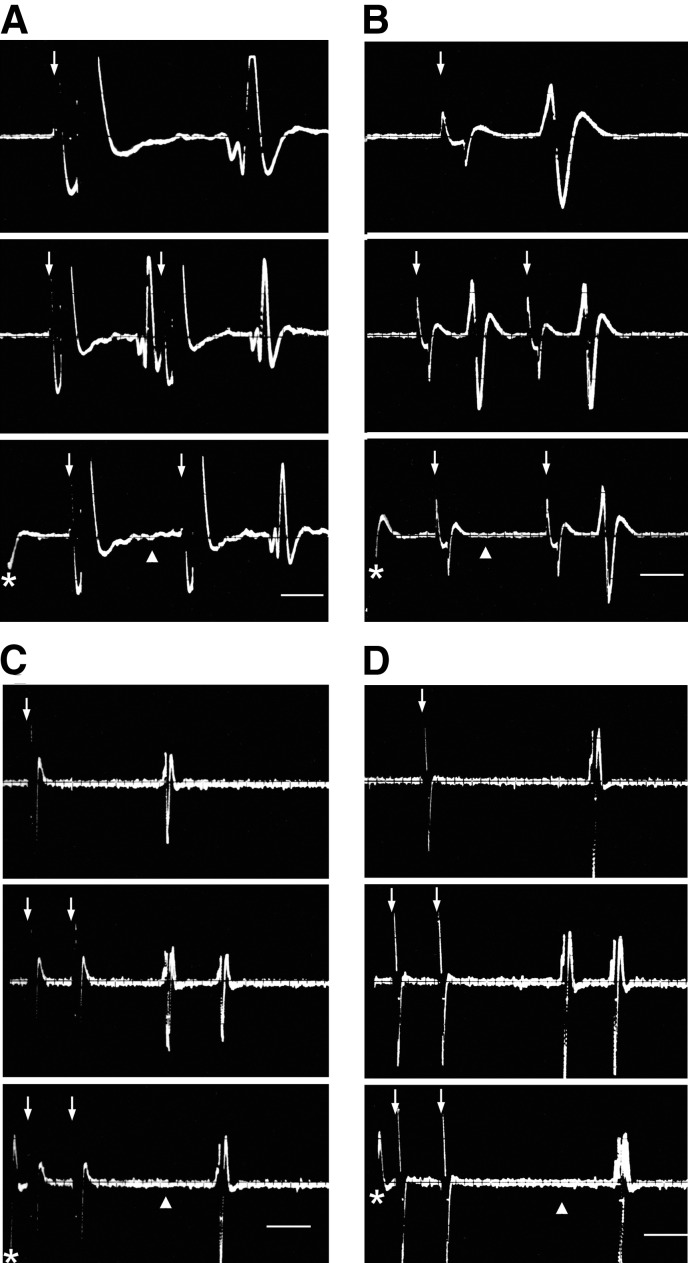
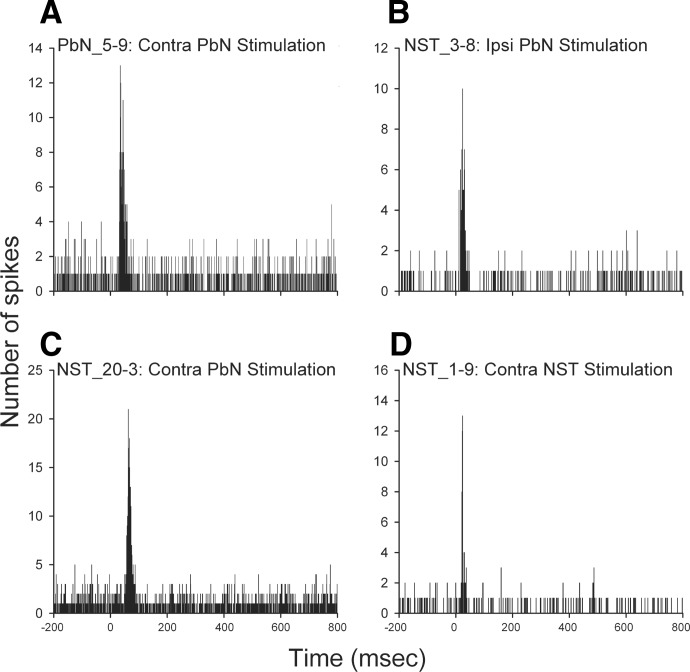
An example of antidromic response of a PbN taste cell following contralateral PbN stimulation appears in Fig. 4A. A PSTH illustrating an orthodromic activation of a PbN taste neuron by stimulation of the contralateral PbN is shown in Fig. 5A.
FIG. 4.
Superimposed oscilloscope traces (n ≧ 3 sweeps) recorded from a taste-responsive PbN cell (A) and 3 NST neurons (B, C, and D), demonstrating fulfillment of criteria for antidromic activation from ipsilateral PbN (B), contralateral PbN (A and C), and contralateral NST (D). Responses occurred at a constant latency to the PbN (A, B, and C) and NST (D) stimuli (arrow, top), followed closely paired stimulation pulses (middle), and were canceled (▴, bottom) by collision with spontaneously generated action potentials (*). The onset latencies for antidromic activation of the units in A, B, C, and D were 3.9, 2.2, 15.2, and 18.8 ms, respectively. Scale bar = 1 ms in A and B, top traces; 2 ms in middle and bottom traces. The scale bars in units C and D represent 5 ms.
FIG. 5.
PSTHs depicting responses of a PbN taste cell (A) and 3 NST gustatory neurons (B, C, and D) following the contralateral PbN (A and C), ipsilateral PbN (B), and contralateral NST (D) stimulation, respectively. Electrical pulses were delivered at time = 0. Each PSTH was accumulated over 200 stimulus sweeps at 1/3 Hz.
Effect of stimulation of the bilateral PbN on NST taste neurons
All 119 taste-responsive NST neurons were examined for their connectivity with ipsilateral and contralateral PbN. Following the ipsilateral PbN stimulation, 110 (92.4%) neurons were activated: 97 (81.5%) projected to the ipsilateral PbN, 13 (10.9%) received projection by synapse from the ipsilateral PbN. Electrical stimulation of the contralateral PbN, on the other hand, activated 49 of 119 (41.2%) NST taste neurons: 4 (3.4%) projected to the contralateral PbN, 45 (37.8%) received projection by synapse from the contralateral PbN. For instance, out of 25 S-best neurons, 24 (96.0%) were ipsilateral PbN-projecting cells (Tables 1 and 2). The gustatory projection from the NST to the ipsilateral PbN was significantly heavier than that to the contralateral PbN (χ2 = 120.6, df = 2, P < 0.001).
Ipsilateral PbN-projection neurons responded more strongly to taste stimuli than the nonprojecting cells: 3.86 ± 0.32 (mean ± SE) impulses/s compared with 2.15 ± 0.31 impulses/s, respectively [F(1,468) = 6.288, P < 0.05], but differences among the four stimuli and interaction between the projection type and taste stimulus were not significant. This result indicates that NST taste neurons carrying taste information to the ipsilateral PbN have greater gustatory sensitivity than that of nonprojection taste cells.
The thresholds to induce antidromic activation of the NST neurons ranged from 12 to 100 and 30 to 100 μA following the ipsilateral and contralateral PbN stimulation, respectively. The onset latencies for antidromic activation ranged from 1.1 to 18.0 ms after ipsilateral PbN stimulation and 3.0 to 15.5 ms following contralateral PbN stimulation, respectively. Although the mean antidromic activation thresholds and latencies were smaller for ipsilateral versus contralateral PbN stimulation, the difference in sample sizes (97 vs. 4) precluded statistical analysis (Table 3). Examples of both ipsi- and contralateral antidromic invasion of NST taste cells appear in Fig. 4, B and C, respectively.
Typically, the threshold for antidromic activation was lower (mean = 55.7 ± 2.5 μA) than the (100-μA) standard used to test for orthodromic responses. Therefore we tested a sample of PbN-projecting NST cells as to whether they also receive input by synapse from the PbN by increasing the PbN stimulus to 100 μA. Of 20 neurons tested, 17 (2 N-best, 5 S-best, 3 C-best, and 7 Q-best neurons) received input by synapse from the ipsilateral PbN at this stimulus intensity, suggesting a majority of NST gustatory neurons not only send taste information to the ipsilateral PbN, but also receive descending input from the same area. NST taste cells received more input via synapse from the ipsilateral PbN than contralateral PbN (χ2 = 13.90, df = 2, P < 0.005).
Orthodromic activation of the NST neurons from the PbN was all excitatory regardless of the side stimulated. The orthodromic response latencies ranged from 7 to 23 ms including ipsilateral PbN-projecting (n = 17) and non-PbN-projecting (n = 13) cells after stimulation of the ipsilateral PbN, and from 19 to 79 ms after stimulation of the contralateral PbN (n = 45). The latencies following stimulation of the contralateral PbN were significantly longer (t = 12.886, df = 73, P < 0.001) than following the ipsilateral PbN stimulation (Table 3). The duration of excitation varied from 8 to 37 and from 8 to 53 ms after the ipsilateral and contralateral PbN stimulation, respectively, and the duration of excitatory response after the contralateral PbN stimulation was also significantly longer (t = 6.933, df = 73, P < 0.001) than after the ipsilateral PbN stimulation (Table 3). Examples of excitatory responses of two NST neurons following ipsilateral and contralateral PbN stimulation are shown in PSTHs in Fig. 5, B and C, respectively.
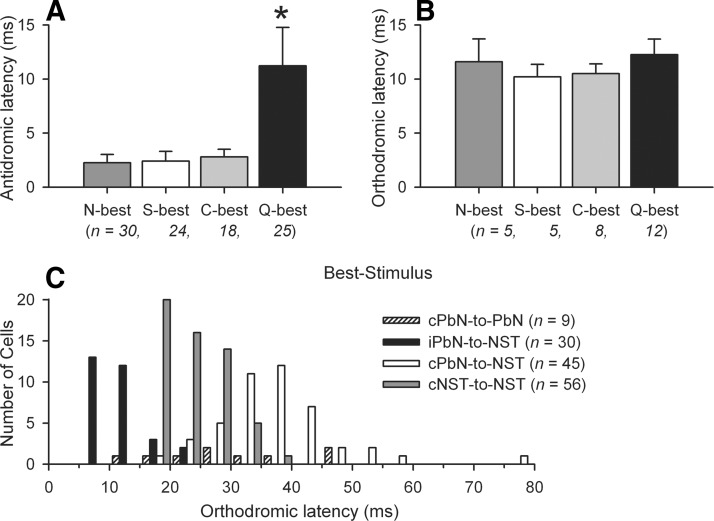
Out of 97 ipsilateral PbN-projecting NST neurons, the latencies of Q-best neurons were significantly longer compared with that of the neurons in other best-stimulus groups [F(3,93) = 125.55, P < 0.001], implying relatively small size of cell bodies or PbN-projecting axons of Q-best cells. In comparison, the orthodromic activation latencies of Q-best NST neurons were not different from those of other best-stimulus groups [F(3,26) = 0.441, P = 0.725]. The means of the all antidromic (n = 97) and a subset of orthodromic (n = 30) activation latencies in each best-stimulus group are shown in Fig. 6, A and B. The thresholds of the ipsilateral PbN-projecting neurons were not different across Q-best and the other best-stimulus groups [F(3,93) = 0.330, P = 0.803]. The distribution of the orthodromic latencies among the four neural connections is compared in Fig. 6C. Out of 45 NST neurons that received input by synapse from the contralateral PbN, 9 cells were not examined for NST–NST connection. Thirty-two of 36 cells also received input by synapse from the contralateral NST. The remaining 4 cells projected to the contralateral NST. The comparison of individual latencies between cPbN-to-NST via synapse and cNST-to-NST via synapse revealed that 11 of 32 cells had latency differences <10 ms (the mean of iPbN-to-NST via synapse was 11.3 ms). The latency of cPbN-to-NST via synapse is correlated to that of cNST-to-NST via synapse (Pearson's r = 0.35, P < 0.05).
FIG. 6.
A: mean antidromic latencies (±SE, ms) of the ipsilateral PbN-projecting NST taste neurons in each best-stimulus group. B: mean orthodromic latencies (±SE, ms) of the 30 NST neurons in each best-stimulus group, in response to the stimulation of the ipsilateral PbN. C: distribution of orthodromic latencies of the PbN and NST taste neurons of 4 neural connections.
Effect of stimulation of the contralateral NST on NST taste neurons
In all, 96 gustatory NST neurons were examined for the influence of electrical stimulation of the contralateral NST. Of those, 66 were activated by contralateral NST stimulation (Table 2); 10 projected to the contralateral NST, 56 received projection by synapse from the contralateral NST (Figs. 4D and 5D). NST taste neurons received more input from the contralateral NST than projected to the contralateral side (χ2 = 33.25, df = 2, P < 0.001) and inter-NST communication was stronger than that of the bilateral PbN (χ2 = 26.22, df = 1, P < 0.001). The antidromic latencies between the NST (mean = 15.9 ± 2.2 ms) were twice that between the bilateral PbN (t = 2.500, df = 13, P < 0.05). All responses of cNST-to-NST via synapse were excitatory. The onset latencies and thresholds for antidromic activation ranged from 8.1 to 30.2 ms and 26 to 93 μA, respectively. The response latencies for orthodromic activation ranged from 15 to 36 ms and the duration of excitation evoked by contralateral NST stimulation varied from 6 to 59 ms (see Table 3 for the means). Each of these 96 NST taste cells was examined for its responsiveness to electrical stimulation of all three nuclei: ipsilateral PbN, contralateral PbN, and contralateral NST. Thirty-five neurons responded to all three sites and 33 cells responded to one site. Table 4 summarizes the number of cells that responded to stimulation of 1, 2, or 3 sites, and the combination of responses.
TABLE 4.
Classification of 96 NST gustatory neurons of which responses were examined to stimulation of three sites as a function of number of effective stimulating sites, response types, and best stimulus
| One Site |
Two Sites |
Three Sites |
||||||
|---|---|---|---|---|---|---|---|---|
| Responses | Number of Cells | Responses | Number of Cells | Responses | Number of Cells | |||
| A-N-N | Nb10, Sb9, Cb5, Qb3 | A-A-N | Sb1 | A-A-A | Sb1 | |||
| E-N-N | Cb1 | A-N-A | Nb2 | A-E-A | Qb1 | |||
| N-N-A | Nb1, Cb1, Qb1 | A-N-E | Nb4, Sb4, Cb6, Qb7 | A-E-E | Nb6, Sb6, Cb5, Qb9 | |||
| N-N-E | Sb1, Cb1 | E-A-N | Nb1 | E-E-A | Cb2 | |||
| E-N-E | Qb1 | E-E-E | Nb1, Cb2, Qb2 | |||||
| N-E-A | Qb1 | |||||||
| N-E-E | Qb1 | |||||||
| Totals | 33 | 28 | 35 | |||||
Order of responses for one site, two sites, and three sites is, respectively, to stimulation of the ipsilateral PbN, contralateral PbN, and contralateral NST. Number following cell type (Nb, Sb, Cb, Qb) indicates the number of cells in that category. A, antidromic activation; Cb, citric acid-best; E, excitatory response; N, no response; Nb, NaCl-best; Sb, sucrose-best; Qb, QHCl-best.
DISCUSSION
The results of the present experiment demonstrated the functional relationships among four gustatory nuclei in the brain stem. The first feature of these relationships was that the afferent gustatory projection from the NST to the PbN is predominantly ipsilateral: the ratio is 82 versus 3%. The second feature was that the connection between the NST and PbN is reciprocal; the majority of gustatory NST neurons receive descending projection from the PbN with an ipsilateral dominance: 80 versus 38%. The third characteristic was that more NST taste neurons received input via synapse from the contralateral NST than projected to the contralateral NST: 59 versus 10%. The fourth point was that inter-PbN communication is less than half of that between the bilateral NST (33 vs. 69%) and more neurons received input via synapse from the contralateral PbN than projected to the contralateral PbN: 21 versus 12%. The brain stem gustatory neural connections examined in the present study together with the brain gustatory neural circuitry that had been revealed previously are illustrated schematically in Fig. 7.
FIG. 7.
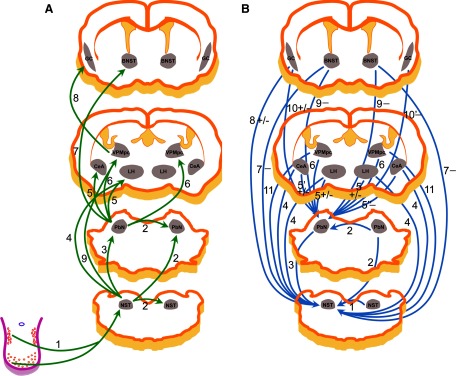
Schematic diagrams illustrating the gustatory neural circuits of the rodent based on electrophysiological investigations. Note that the diagrams were based on the left (ipsilateral) side of the networks. A: projections from the recorded neuron to the stimulating site: 1: Li et al. 2008; Norgren et al. 1989; Smith et al. 1983; 2: present experiment; 3: Cho et al. 2002a; Monroe and Di Lorenzo 1995; Ogawa et al. 1984b, present experiment; 4: Cho et al. 2002b, 2003; 5: Li et al. 2005; Norgren 1974, 1976; 6: Hayama and Ogawa 1987; Mao et al. 2008; Norgren 1974, 1976; 7: Li and Cho 2006; 8: Ogawa and Nomura 1988; 9: Cho et al. 2008. B: projections from the stimulating site to the recorded neuron via synapse(s): 1: Li et al. 2008, present experiment; 2: present experiment; 3: Cho et al. 2002a, present experiment; 4: Cho et al. 2002b, 2003; Li et al. 2002; 5: Li et al. 2005; Lundy Jr and Norgren 2004a; 5′: Li et al. 2005; Lundy Jr and Norgren 2001, 2004a; 5": Lundy Jr and Norgren 2004a; 6: Mao et al. 2008; 7: Smith et al. 2005; 8: Smith and Li 2000; 9: Li and Cho 2006; 10: Di Lorenzo and Monroe 1992; Lundy Jr and Norgren 2004a; 10′: Lundy and Norgren 2004a; 11: Cho et al. 2008. The stimulation effects are indicated as follows: [−] if inhibitory responses were exclusively (9) or dominant (5", 7, 10′), [+/−] if excitatory and inhibitory responses were comparable (5, 8) or reported in different studies (5′, 10), and no symbol if the excitatory responses were exclusively (1, 2, 3) or prevailing (4, 6, 11).
Reciprocal connections of NST taste neurons with bilateral gustatory PbN
The present study has demonstrated that taste neurons in the NST send their axons to the bilateral PbN and that both sides of the PbN send projections back to influence activity of taste neurons in the NST. The majority (82%) of NST taste neurons projected to the ipsilateral PbN. This proportion of solitario-parabrachial relay neurons is consistent with a previous report in the hamster (Cho et al. 2002a) but higher than that in rats (31–45%) (Geran and Travers 2006; Monroe and Di Lorenzo 1995; Ogawa and Kaisaku 1982; Ogawa et al. 1980, 1984b). One of the factors that might contribute to the difference in the projection ratio between the hamster and rat is the difference in number of S-best cells identified in the NST between these two species. For example, 25 of 119 (21%) cells recorded in the present study were S-best, whereas far fewer S-best cells were found in the rat (5 of 40, 13%) (Monroe and Di Lorenzo 1995). Much higher proportions of S-best cells projected to the PbN both in the hamster (24 of 25, 96%, the present data) and rat (4 of 5, 80%) (Monroe and Di Lorenzo 1995).
In addition to the ipsilateral projection, the present data revealed that a few NST taste neurons project to the contralateral PbN, a connection that was not investigated in previous studies (Cho et al. 2002a; Monroe and Di Lorenzo 1995; Ogawa et al. 1984b). This finding is consistent with anatomical obervations. Neuroanatomical studies have also demonstrated the projection of the rostral NST cells to the contralateral PbN in the rat and hamster (Whitehead et al. 2000; Williams et al. 1996).
Previous investigations in the hamster showed that a few NST taste neurons projected to the ipsilateral lateral hypothalamus (LH), but not to the contralateral LH; bilateral central nucleus of the amygdala (CeA); or bed nucleus of the stria terminalis (BNST) (Cho et al. 2002b, 2003; Li et al. 2002; Smith et al. 2005). In contrast, a substantial proportion of the gustatory PbN cells send axons to the LH, CeA, or VPMpc in the hamster or in the rat (Bester et al. 1997; Hayama and Ogawa 1987; Li et al. 2005; Mao et al. 2008; Norgren 1976; Norgren and Leonard 1973). These results confirm that the majority of taste information is carried to the ipsilateral PbN from the gustatory NST before it is further transferred to the higher taste centers in the rodent.
Whereas axonal projection from NST taste neurons to the PbN was predominantly ipsilateral, descending input from the PbN was bilateral, but not equally distributed: 80% ipsi versus 38% contra. We previously reported that a subset of non-PbN-projecting taste neurons in the NST receive centrifugal input from the ipsilateral PbN in the hamster, although we did not test PbN-projecting NST neurons in that study (Cho et al. 2002a). The present results added two new findings to the previous studies. The first is that not only non-PbN-projecting NST cells but some PbN-projecting NST cells also receive descending input from the ipsilateral PbN. Therefore a higher proportion of NST cells was confirmed to be under descending influence from the ipsilateral PbN. If we assumed that the same proportion of the entire 97 ipsilateral PbN-projecting NST cells received descending input by synapse from the ipsilateral PbN as those tested (17 of 20), then 82 PbN-projecting cells would have received descending input by synapse from the ipsilateral PbN. Thus 80% of NST taste cells might receive descending input from the ipsilateral PbN, including the PbN-projecting and non-PbN-projecting neurons. It had been demonstrated that a sizable number of NST taste neurons received centrifugal input from the ventral forebrain. Stimulation of the ipsilateral LH, CeA, BNST, and gustatory cortex modulated the activity of 19, 20, 25, and 34% of taste neurons in the hamster NST, respectively (Cho et al. 2002b; Li et al. 2002; Smith and Li 2000; Smith et al. 2005). The second is that taste neurons in the NST also receive sizable centrifugal input from the contralateral PbN. The intensity of this centrifugal projection (38%) was similar to that from the contralateral LH (41%) but greater than that from the contralateral CeA (26%) or BNST (15%) (Cho et al. 2002b; Li et al. 2002; Smith et al. 2005). The centrifugal input from both sides of the PbN to NST cells was exclusively excitatory, whereas modulation of NST taste cells from the ventral forebrain was both excitatory and inhibitory (Cho et al. 2002b; Li et al. 2002; Smith and Li 2000; Smith et al. 2005).
Possible centrifugal neural pathways from the PbN-to-NST taste neurons
Three possible mechanisms explain modulatory input to NST neurons from the PbN: monosynaptic, fibers of passage, and indirect loops. The simplest is a direct, monosynaptic connection. The electrical stimulation could activate cell bodies in the PbN that send axons to the NST. In this case, the long orthodromic latencies imply very small axons with slow conduction velocities well below 0.1 m/s. An anatomical basis for such an influence was revealed by tracer injections into the gustatory PbN that labeled fibers in the rostral NST and medullary reticular formation in rats (Karimnamazi and Travers 1998). A second possibility—that the effect was due to the activation of fibers of passage—cannot be excluded, but seems unlikely because the main contingent of such axons, in the brachium conjunctivum, are myelinated and project rostrally. Finally, NST neurons could receive input from the PbN via multisynaptic loops. The long latencies of some of the efferent projections from the contralateral PbN to the taste cells in the NST could be most easily explained by this model. The gustatory PbN projects to the VPMpc and, separately, to the ventral forebrain nuclei (Halsell 1992; Hayama and Ogawa 1987; Jhamandas et al. 1996; Karimnamazi and Travers 1998; Norgren 1974, 1976; Norgren and Leonard 1973; Saper and Loewy 1980). Ventral forebrain nuclei send axons back to the rostral NST (Cho et al. 2002b, 2003; Dong and Swanson 2004; Li et al. 2002; Smith et al. 2005; van der Kooy et al. 1984; Whitehead et al. 2000). Therefore it is possible that stimulating of the PbN activated neurons in the target nuclei of the PbN projection in the ventral forebrain, and then NST taste neurons. Such multisynaptic loops could exist within the brain stem itself because the rostral and caudal NST, the PbN, and medullary reticular formation are anatomically connected (Beckman and Whitehead 1991; Halsell et al. 1996; Karimnamazi and Travers 1998; Streefland and Jansen 1999; Ter Horst et al. 1991; Travers and Hu 2000). With the present data, we could not rule out any of these mechanisms.
There are several possible routes within the brain stem that connect the contralateral PbN and NST via synapse. For example, the route may pass through contralateral NST before it reaches the NST. This possibility was supported by the fact that the majority of the NST cells that received input from the contralateral PbN also received input from the contralateral NST. Furthermore, the latencies of cPbN-to-NST via synapse were correlated to those of cNST-to-NST via synapse. However, latency differences between cPbN-to-NST and cNST-to-NST were <10 ms in 11 of 32 NST cells (mean of iPbN-to-NST was 11.3 ms) that received descending input from the contralateral PbN and NST. It may suggest that the possibility of involvement of the contralateral NST is minimal in these cells. Experiments that involve reversibly blocking bilateral PbN/contralateral NST are warranted to further clarify this neural connection.
Reciprocal connections between NSTs versus between PbNs
We demonstrated that the neuronal activity of brain stem taste neurons in the NST and PbN was modulated by stimulation of their contralateral counterparts. Stimulation of the contralateral NST activated 66 of 96 NST cells: 10 cells projected directly to and 56 cells received input by synapse from the contralateral NST. These results suggest that more than half of the taste neurons in the NST receive gustatory information from both sides. Whitehead and colleagues (2000) reported that following microinjection of cholera toxin B into the rostral NST, both labeled neurons and axons were found in the rostral central and ventral subdivisions in the contralateral NST. The rostral central subnucleus corresponded to the recording marks identified in the present study. Among 10 NST cells that projected to the contralateral NST, 4 were also ipsilateral PbN-projecting cells; one other cell, S-best, projected to all three sites. In a recent study we found that a subset of NST taste neurons responded to electrical stimulation of the contralateral chorda tympani (CT) nerve and that these responses could be blocked by local anesthetization of the contralateral NST (Li et al. 2008). Furthermore, taste responses of NST cells were reduced by anesthetization of the contralateral NST in that study. Taken together, these results imply that NST neurons receive taste information from ipsilateral primary afferent synapses and via second-order projections from the contralateral side.
Similarly, PbN gustatory neurons responded to the stimulation of the contralateral PbN. The gustatory neural connection between PbNs was also supported neuroanatomically: following the microinjection of wheat germ agglutinin–horseradish peroxidase into the PbN, many retrogradely labeled cells were found in the contralateral PbN, particularly in the ventral lateral, central lateral, and in Kolliker–Fuse subnuclei in the rat (Moga et al. 1990). The direct axonal projections of PbN neurons to the contralateral PbN occurred with a frequency similar to that between the bilateral NST: 12 versus 10%. The PbN cells that receive input by synapse from the contralateral PbN occurred less frequently than between the bilateral NST: 21 versus 59%. The antidromic latencies between the NST were twice that between the bilateral PbN (mean = 15.9 vs. 7.5 ms) in spite of the similar internuclear distances: the typical measurement in hamsters in the present experiments was 2.6 ms between the bilateral NST versus 2.8 ms between the bilateral PbN. It may indicate differences in axon size between inter-PbN and inter-NST connections or that these internuclear connections take different routes to reach the contralateral side.
The present experiments document the complexity of neural connections among taste-responsive neurons in the brain stem. Gustatory neurons in the NST project directly to at least one of the three other brain stem taste nuclei and in some cases receive transynaptic feedback from their synaptic targets as well.
Characteristics of taste neurons in the brain stem gustatory nuclei
In the present study, each gustatory neuron was classified into one of four best-stimulus groups based on the responses to the four basic taste stimuli. The number of cells or their entropy did not differ across the best-stimulus categories. Nonetheless, Q-best neurons had a distinct feature. Among the 97 ipsilateral PbN-projecting neurons, the latencies of Q-best cells were significantly longer than those of the other best-stimulus groups. The shortest latency of Q-best NST neurons was 5.5 ms, whereas the longest latency of non-Q-best cells was 4.4 ms. This result is consistent with our previous data (Cho et al. 2002a) and may suggest that Q-best NST neurons are smaller and have thinner axons than those of the other cell types because conduction velocity (or latency) is related to the size of the cell or the axon (Gasser and Grundfest 1939; Harper and Lawson 1985). Furthermore, more Q-best cells in both the NST and PbN received input by synapse from other brain stem nuclei (Table 2). Among 96 NST neurons that were tested for response to the stimulation of the other three nuclei, Q-best cells had a tendency to respond to multiple gustatory sites: 22 of 26 responded to the stimulation of two or three gustatory nuclei (Table 4). These data indicate that Q-best neurons have a greater connectivity between brain stem gustatory nuclei than that of other best-stimulus types in the hamster.
Taste responses of NST neurons that projected to the ipsilateral PbN were greater than those of NST cells that did not project to the same area. A similar difference was also reported in previous studies using the hamster and rat (Cho et al. 2002a; Ogawa et al. 1984b). In the rat PbN, gustatory responses of the parabrachio-thalamic taste neurons were also greater than those of nonprojecting cells (Ogawa et al. 1987). In the present study, all but one of the S-best neurons projected to the ipsilateral PbN. Again, parallel results have been reported previously (Ogawa et al. 1984b). Finally, a more robust sweet response was observed in NST cells that project to the PbN (Monroe and Di Lorenzo 1995). Taken together, these results suggest that, although the projections of NST neurons are not associated with their taste-response profile, pleasant taste information is transferred further rostrally, whereas more of the aversive taste information may be processed within the brain stem gustatory nuclei.
Physiological implication
The demonstration of the presence of reciprocal neural connections among the brain stem gustatory nuclei provides the foundation for further understanding of the neuronal basis for the processing, integration, and modulation of gustatory information within the brain stem. Two taste relays in the hamster brain stem are in fact cross-connected, and that feedback projects both ipsi- and contralaterally. Ipsilaterally, the proportions of axonal projections of the cells in both the NST (82%, present data) and PbN (91%; Mao et al. 2008) to the second and third central relays are extensive and approximately the same in the hamster. Such high levels of gustatory afferent projections may be beneficial in ensuring the conveyance of gustatory information to the cortex. Cells in both the NST and PbN also project to the corresponding contralateral nucleus as well as to the next taste nuclei. This divergent projection may be favorable in ensuring both sides of gustatory nuclei receive gustatory information at the level of the first and second central relays in the circumstance that only a small amount of taste is presented on one side of the tongue or unilateral taste input is blocked peripherally. We have previously shown that the NST cells receive convergent gustatory information from both sides of the tongue (Li et al. 2008). Such organization of neural networks has also been implicated in the amplification of neuronal activity. The activity of cells in the orbital and medial prefrontal cortex was greatly augmented by simultaneous activation of hippocampal and amygdalar neurons (Ishikawa and Nakamura 2003). Finch (1996) reported that simultaneous stimulation of more than one brain site, such as thalamus, amygdala, and subiculum/CA1, produced spatial and temporal summation to increase the probability of spike firing of striatal cells. Indeed, our previous study has shown that taste responses were decreased after blocking the input from the contralateral CT (Li et al. 2008). The proportion of efferent projections from the third gustatory relay to the cells in the PbN (89%; Mao et al. 2008) is similar to that from the second gustatory relay to the cells in the NST (80%,; present data). Besides, cells in both the NST and PbN receive convergent efferent inputs from multiple gustatory nuclei with the cells in the PbN receiving more convergent inputs including the VPMpc (Mao et al. 2008), BNST (Li and Cho 2006), LH, CeA, and gustatory cortex (Di Lorenzo and Monroe 1992; Li and Cho 2006; Li et al. 2005; Lundy Jr and Norgren 2001, 2004a; Mao et al. 2008). The NST and PbN are known as the sites where integration of gustatory information and other visceral sensory inputs—for example, gastric distention/duodenal lipid-evoked visceral sensory information—take place (Baird et al. 2001a,b; Glenn and Erickson 1976; Hajnal et al. 1999). Therefore it is favorable for cells in the PbN and NST to receive more diverse information.
The PbN is a critical neural substrate in acquiring conditioned taste aversion (CTA) and forebrain taste relays that send projections to the PbN and/or NST are also involved in the CTA (Reilly and Trifunovic 2000; Scalera et al. 1995; Schafe and Bernstein 1996, 1998; Spector et al. 1992). Some researchers have suggested that the circuit used for producing CTA is lateralized; the lesions of PbN and ipsilateral amygdala did not prevent acquisition of CTA, whereas the lesions of the PbN and contralateral amygdala did (Bielavska and Roldan 1996; Schafe and Bernstein 1996). These experiments suggest that CTA requires intact neural connection in the same hemisphere. It is consistent with neuroanatomical observations. Previous studies demonstrated that the afferent projections of the cells in the gustatory NST, PbN, and CeA are predominantly ipsilateral (Halsell 1992; Norgren and Leonard 1973; Saper and Loewy 1980; Whitehead et al. 2000), suggesting that the ipsilateral afferent projection may play a major role in taste information processing and CTA. Indeed, the present study also shows that taste neurons in the NST project predominantly to the ipsilateral PbN. Descending projections to the contralateral gustatory nuclei (e.g., descending projection from the contralateral CeA to the taste cells in the PbN) may play a complementary role in CTA (Lundy Jr 2004).
GRANTS
This work was supported in part by National Institute on Deafness and Other Communication Disorders Grant DC-006623 to C.-S. Li.
Acknowledgments
We thank Dr. Ralph Norgren for valuable comments on an earlier draft of the manuscript and Dr. Andrew Sharp for instructive comments on the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Angel and Gratton 1982.Angel A, Gratton DA. The effect of anaesthetic agents on cerebral cortical responses in the rat. Br J Pharmacol 76: 541–549, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird et al. 2001a.Baird JP, Travers JB, Travers SP. Parametric analysis of gastric distension responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1568–R1580, 2001a [DOI] [PubMed] [Google Scholar]
- Baird et al. 2001b.Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1581–R1593, 2001b [DOI] [PubMed] [Google Scholar]
- Beckman and Whitehead 1991.Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res 557: 265–279, 1991 [DOI] [PubMed] [Google Scholar]
- Bester et al. 1997.Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 383: 245–281, 1997 [DOI] [PubMed] [Google Scholar]
- Bielavska and Roldan 1996.Bielavska E, Roldan G. Ipsilateral connections between the gustatory cortex, amygdala and parabrachial nucleus are necessary for acquisition and retrieval of conditioned taste aversion in rats. Behav Brain Res 81: 25–31, 1996 [DOI] [PubMed] [Google Scholar]
- Cho et al. 2002a.Cho YK, Li C-S, Smith DV. Gustatory projections from the nucleus of the solitary tract to the parabrachial nuclei in the hamster. Chem Senses 27: 81–90, 2002a [DOI] [PubMed] [Google Scholar]
- Cho et al. 2002b.Cho YK, Li C-S, Smith DV. Taste responses of neurons of the hamster solitary nucleus are enhanced by lateral hypothalamic stimulation. J Neurophysiol 87: 1981–1992, 2002b [DOI] [PubMed] [Google Scholar]
- Cho et al. 2003.Cho YK, Li C-S, Smith DV. Descending influences from the lateral hypothalamus and amygdala converge onto medullary taste neurons. Chem Senses 28: 155–171, 2003 [DOI] [PubMed] [Google Scholar]
- Cho et al. 1991.Cho YK, Mao L, Li C-S. Modulation of solitary taste neurons by electrical stimulation of the ventroposteromedial nucleus of the thalamus in the hamster. Brain Res 10.1016/j.brainres.2008.05.006 [DOI] [PubMed]
- Davis 1991.Davis BJ. The ascending gustatory pathway: a Golgi analysis of the medial and lateral parabrachial complex in the adult hamster. Brain Res Bull 27: 63–73, 1991 [DOI] [PubMed] [Google Scholar]
- Davis and Jang 1988.Davis BJ, Jang T. A Golgi analysis of the gustatory zone of the nucleus of the solitary tract in the adult hamster. J Comp Neurol 278: 388–396, 1988 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo and Monroe 1992.Di Lorenzo PM, Monroe S. Corticofugal input to taste-responsive units in the parabrachial pons. Brain Res Bull 29: 925–930, 1992 [DOI] [PubMed] [Google Scholar]
- Dong and Swanson 2004.Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol 468: 277–298, 2004 [DOI] [PubMed] [Google Scholar]
- Erickson 1966.Erickson RP. Non-traumatic headholders for mammals. Physiol Behav 1: 97–98, 1966 [Google Scholar]
- Finch 1996.Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus 6: 495–512, 1996 [DOI] [PubMed] [Google Scholar]
- Gasser and Grundfest 1939.Gasser HS, Grundfest H. Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian A fibers. Am J Physiol—Legacy Content 127: 393–414, 1939 [Google Scholar]
- Geran and Travers 2006.Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol 96: 2513–2527, 2006 [DOI] [PubMed] [Google Scholar]
- Glenn and Erickson 1976.Glenn JF, Erickson RP. Gastric modulation of gustatory afferent activity. Physiol Behav 16: 561–568, 1976 [DOI] [PubMed] [Google Scholar]
- Hajnal et al. 1999.Hajnal A, Takenouchi K, Norgren R. Effect of intraduodenal lipid on parabrachial gustatory coding in awake rats. J Neurosci 19: 7182–7190, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsell 1992.Halsell CB. Organization of parabrachial nucleus efferents to the thalamus and amygdala in the golden hamster. J Comp Neurol 317: 57–78, 1992 [DOI] [PubMed] [Google Scholar]
- Halsell and Frank 1991.Halsell CB, Frank ME. Mapping study of the parabrachial taste-responsive area for the anterior tongue in the golden hamster. J Comp Neurol 306: 708–722, 1991 [DOI] [PubMed] [Google Scholar]
- Halsell and Frank 1992.Halsell CB, Frank ME. Organization of taste-evoked activity in the hamster parabrachial nucleus. Brain Res 572: 286–290, 1992 [DOI] [PubMed] [Google Scholar]
- Halsell et al. 1996.Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience 72: 185–197, 1996 [DOI] [PubMed] [Google Scholar]
- Harper and Lawson 1985.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 359: 31–46, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama and Ogawa 1987.Hayama T, Ogawa H. Electrophysiological evidence of collateral projections of parabrachio-thalamic relay neurons. Neurosci Lett 83: 95–100, 1987 [DOI] [PubMed] [Google Scholar]
- Ishikawa and Nakamura 2003.Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci 23: 9987–9995, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas et al. 1996.Jhamandas JH, Petrov T, Harris KH, Vu T, Krukoff TL. Parabrachial nucleus projection to the amygdala in the rat: electrophysiological and anatomical observations. Brain Res Bull 39: 115–126, 1996 [DOI] [PubMed] [Google Scholar]
- Karimnamazi and Travers 1998.Karimnamazi H, Travers JB. Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Res 813: 283–302, 1998 [DOI] [PubMed] [Google Scholar]
- Li and Cho 2006.Li C-S, Cho YK. Efferent projection from the bed nucleus of the stria terminalis suppresses activity of taste-responsive neurons in the hamster parabrachial nuclei. Am J Physiol Regul Integr Comp Physiol 291: R914–R926, 2006 [DOI] [PubMed] [Google Scholar]
- Li et al. 2002.Li C-S, Cho YK, Smith DV. Taste responses of neurons in the hamster solitary nucleus are modulated by the central nucleus of the amygdala. J Neurophysiol 88: 2979–2992, 2002 [DOI] [PubMed] [Google Scholar]
- Li et al. 2005.Li C-S, Cho YK, Smith DV. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol 93: 1183–1196, 2005 [DOI] [PubMed] [Google Scholar]
- Li et al. 2008.Li C-S, Mao L, Cho YK. Taste-responsive neurons in the nucleus of the solitary tract receive gustatory information from both sides of the tongue in the hamster. Am J Physiol Regul Integr Comp Physiol 294: R372–R381, 2008 [DOI] [PubMed] [Google Scholar]
- Lundy and Norgren 2001.Lundy RF Jr, Norgren R. Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J Neurophysiol 85: 770–783, 2001 [DOI] [PubMed] [Google Scholar]
- Lundy and Norgren 2004a.Lundy RF Jr, Norgren R. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol 91: 1143–1157, 2004a [DOI] [PubMed] [Google Scholar]
- Lundy and Norgren 2004b.Lundy RF Jr, Norgren R. Gustatory system. In: The Rat Nervous Systemm, edited by Paxinos G. Amsterdam: Elsevier/Academic, 2004b, p. 891–921.
- Mao et al. 2008.Mao L, Cho YK, Li C-S. Modulation of activity of gustatory neurons in the hamster parabrachial nuclei by electrical stimulation of the ventroposteromedial nucleus of the thalamus. Am J Physiol Regul Integr Comp Physiol 294: R1461–R1473, 2008 [DOI] [PubMed] [Google Scholar]
- McPheeters et al. 1990.McPheeters M, Hettinger TP, Nuding SC, Savoy LD, Whitehead MC, Frank ME. Taste-responsive neurons and their locations in the solitary nucleus of the hamster. Neuroscience 34: 745–758, 1990 [DOI] [PubMed] [Google Scholar]
- Moga et al. 1990.Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol 295: 624–661, 1990 [DOI] [PubMed] [Google Scholar]
- Monroe and Di Lorenzo 1995.Monroe S, Di Lorenzo PM. Taste responses in neurons in the nucleus of the solitary tract that do and do not project to the parabrachial pons. J Neurophysiol 74: 249–257, 1995 [DOI] [PubMed] [Google Scholar]
- Morin and Wood 2001.Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. San Diego, CA: Academic Press, 2001, p. 216
- Norgren 1974.Norgren R. Gustatory afferents to ventral forebrain. Brain Res 81: 285–295, 1974 [DOI] [PubMed] [Google Scholar]
- Norgren 1976.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol 166: 17–30, 1976 [DOI] [PubMed] [Google Scholar]
- Norgren 1978.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience 3: 207–218, 1978 [DOI] [PubMed] [Google Scholar]
- Norgren and Leonard 1973.Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol 150: 217–237, 1973 [DOI] [PubMed] [Google Scholar]
- Norgren et al. 1989.Norgren R, Nishijo H, Travers SP. Taste responses from the entire gustatory apparatus. Ann NY Acad Sci 575: 246–263; discussion 263–244, 1989 [DOI] [PubMed] [Google Scholar]
- Norgren and Pfaffmann 1975.Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res 91: 99–117, 1975 [DOI] [PubMed] [Google Scholar]
- Ogawa et al. 1984a.Ogawa H, Hayama T, Ito S. Location and taste responses of parabrachio-thalamic relay neurons in rats. Exp Neurol 83: 507–517, 1984a [DOI] [PubMed] [Google Scholar]
- Ogawa et al. 1987.Ogawa H, Hayama T, Ito S. Response properties of the parabrachio-thalamic taste and mechanoreceptive neurons in rats. Exp Brain Res 68: 449–457, 1987 [DOI] [PubMed] [Google Scholar]
- Ogawa et al. 1980.Ogawa H, Imoto T, Hayama T. Taste relay neurons in the solitary tract nucleus of rats. Neurosci Lett 18: 295–299, 1980 [DOI] [PubMed] [Google Scholar]
- Ogawa et al. 1984b.Ogawa H, Imoto T, Hayama T. Responsiveness of solitario-parabrachial relay neurons to taste and mechanical stimulation applied to the oral cavity in rats. Exp Brain Res 54: 349–358, 1984b [DOI] [PubMed] [Google Scholar]
- Ogawa and Kaisaku 1982.Ogawa H, Kaisaku J. Physiological characteristics of the solitario-parabrachial relay neurons with tongue afferent inputs in rats. Exp Brain Res 48: 362–368, 1982 [DOI] [PubMed] [Google Scholar]
- Ogawa and Nomura 1988.Ogawa H, Nomura T. Receptive field properties of thalamo-cortical taste relay neurons in the parvicellular part of the posteromedial ventral nucleus in rats. Exp Brain Res 73: 364–370, 1988 [DOI] [PubMed] [Google Scholar]
- Reilly and Trifunovic 2000.Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: aversive and appetitive gustatory conditioning. Brain Res Bull 52: 269–278, 2000 [DOI] [PubMed] [Google Scholar]
- Renehan et al. 1994.Renehan WE, Jin Z, Zhang X, Schweitzer L. Structure and function of gustatory neurons in the nucleus of the solitary tract. I. A classification of neurons based on morphological features. J Comp Neurol 347: 531–544, 1994 [DOI] [PubMed] [Google Scholar]
- Renehan et al. 1996.Renehan WE, Jin Z, Zhang X, Schweitzer L. Structure and function of gustatory neurons in the nucleus of the solitary tract: II. Relationships between neuronal morphology and physiology. J Comp Neurol 367: 205–221, 1996 [DOI] [PubMed] [Google Scholar]
- Saper and Loewy 1980.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res 197: 291–317, 1980 [DOI] [PubMed] [Google Scholar]
- Scalera et al. 1995.Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci 109: 997–1008, 1995 [PubMed] [Google Scholar]
- Schafe and Bernstein 1996.Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion: I. The amygdala. Brain Res 741: 109–116, 1996 [DOI] [PubMed] [Google Scholar]
- Schafe and Bernstein 1998.Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion. II. Insular (gustatory) cortex. Brain Res 800: 40–47, 1998 [DOI] [PubMed] [Google Scholar]
- Shirasaka and Wasterlain 1995.Shirasaka Y, Wasterlain CG. The effect of urethane anesthesia on evoked potentials in dentate gyrus. Eur J Pharmacol 282: 11–17, 1995 [DOI] [PubMed] [Google Scholar]
- Smith and Bealer 1976.Smith DV, Bealer SL. Recovery of excitability after gustatory adaptation: effects of stimulus intensity. Sens Processes 1: 99–108, 1976 [PubMed] [Google Scholar]
- Smith and Li 2000.Smith DV, Li C-S. GABA-mediated corticofugal inhibition of taste-responsive neurons in the nucleus of the solitary tract. Brain Res 858: 408–415, 2000 [DOI] [PubMed] [Google Scholar]
- Smith and Travers 1979.Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses Flav 4: 215–229, 1979 [Google Scholar]
- Smith et al. 1983.Smith DV, Van Buskirk RL, Travers JB, Bieber SL. Gustatory neuron types in hamster brain stem. J Neurophysiol 50: 522–540, 1983 [DOI] [PubMed] [Google Scholar]
- Smith et al. 2005.Smith DV, Ye MK, Li C-S. Medullary taste responses are modulated by the bed nucleus of the stria terminalis. Chem Senses 30: 421–434, 2005 [DOI] [PubMed] [Google Scholar]
- Spector et al. 1992.Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci 106: 147–161, 1992 [DOI] [PubMed] [Google Scholar]
- Streefland and Jansen 1999.Streefland C, Jansen K. Intramedullary projections of the rostral nucleus of the solitary tract in the rat: gustatory influences on autonomic output. Chem Senses 24: 655–664, 1999 [DOI] [PubMed] [Google Scholar]
- Ter Horst et al. 1991.Ter Horst GJ, Copray JC, Liem RS, Van Willigen JD. Projections from the rostral parvocellular reticular formation to pontine and medullary nuclei in the rat: involvement in autonomic regulation and orofacial motor control. Neuroscience 40: 735–758, 1991 [DOI] [PubMed] [Google Scholar]
- Travers 1988.Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res 457: 1–11, 1988 [DOI] [PubMed] [Google Scholar]
- Travers and Hu 2000.Travers SP, Hu H. Extranuclear projections of rNST neurons expressing gustatory-elicited Fos. J Comp Neurol 427: 124–138, 2000 [PubMed] [Google Scholar]
- Travers and Smith 1984.Travers SP, Smith DV. Responsiveness of neurons in the hamster parabrachial nuclei to taste mixtures. J Gen Physiol 84: 221–250, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk and Smith 1981.Van Buskirk RL, Smith DV. Taste sensitivity of hamster parabrachial pontine neurons. J Neurophysiol 45: 144–171, 1981 [DOI] [PubMed] [Google Scholar]
- van der Kooy et al. 1984.van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol 224: 1–24, 1984 [DOI] [PubMed] [Google Scholar]
- Voshart and van der Kooy 1981.Voshart K, van der Kooy D. The organization of the efferent projections of the parabrachial nucleus of the forebrain in the rat: a retrograde fluorescent double-labeling study. Brain Res 212: 271–286, 1981 [DOI] [PubMed] [Google Scholar]
- Whitehead 1988.Whitehead MC. Neuronal architecture of the nucleus of the solitary tract in the hamster. J Comp Neurol 276: 547–572, 1988 [DOI] [PubMed] [Google Scholar]
- Whitehead 1990.Whitehead MC. Subdivisions and neuron types of the nucleus of the solitary tract that project to the parabrachial nucleus in the hamster. J Comp Neurol 301: 554–574, 1990 [DOI] [PubMed] [Google Scholar]
- Whitehead et al. 2000.Whitehead MC, Bergula A, Holliday K. Forebrain projections to the rostral nucleus of the solitary tract in the hamster. J Comp Neurol 422: 429–447, 2000 [PubMed] [Google Scholar]
- Whitehead and Frank 1983.Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol 220: 378–395, 1983 [DOI] [PubMed] [Google Scholar]
- Williams et al. 1996.Williams JB, Murphy DM, Reynolds KE, Welch SJ, King MS. Demonstration of a bilateral projection from the rostral nucleus of the solitary tract to the medial parabrachial nucleus in rat. Brain Res 737: 231–237, 1996 [DOI] [PubMed] [Google Scholar]