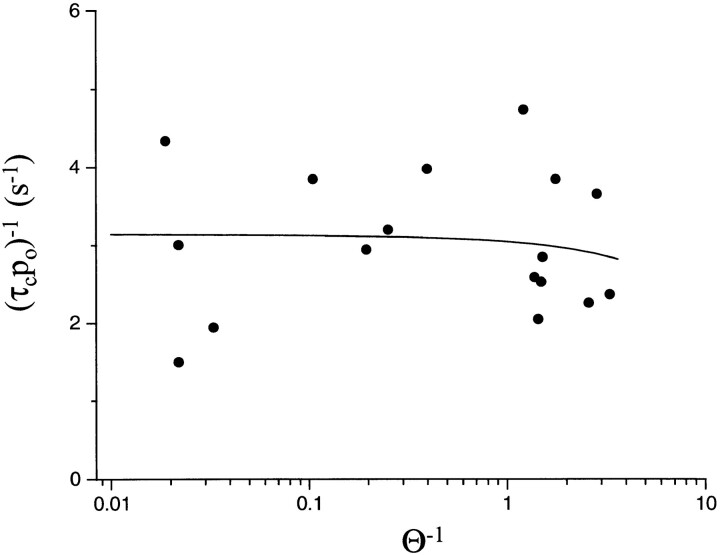
Figure 4.
The desensitization rate constant, (τc P o)−1, for diliganded AChR does not change with the gating equilibrium constant (θ = β/α; see Model I). θ was varied experimentally by using different receptors, agonists, and membrane potentials. The line is the fit by Eq. 5 with k A2O +D = 3.13 s−1 and k A2C +D = −0.08 s−1. Over a ∼1,000-fold range in θ, the slope of the line is indistinguishable from zero, indicating that diliganded-open AChR desensitize much faster than diliganded-closed AChR. This suggests that desensitization is a function of the status of the activation gate rather than the occupancy of the binding sites. Each symbol is the average value for a α2βδε receptor (n patches) (wt [11], αY93F [15], αW149W [4], αG153S [5], εD175N [3], αY198F [10], εE181Q [5], εE184A [4], and I [4]; see Table II) activated under a variety of experimental conditions of agonist (ACh, TMA, CCh), membrane potential (−50, −75, −100, and −130 mV, ) and extracellular salt solution (115 mM NaCl, 140 mM KCl). A total of 61 patches are represented in the plot.