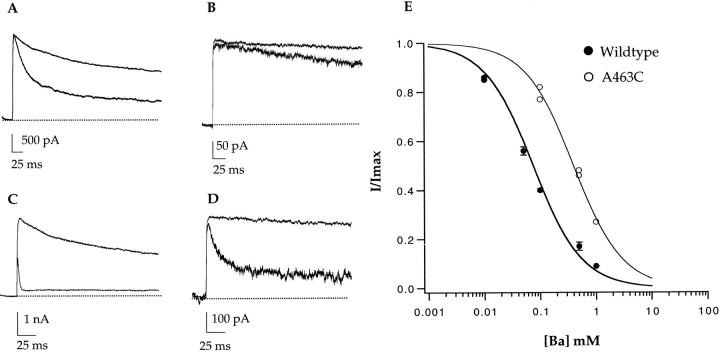
Figure 9.
A463C decreases the internal Ba++ affinity of the channel. Internal Ba++ affinity was measured in both the wild-type channel and A463C with steps to +100 mV in the presence of symmetrical 140 mM KCl. Wild-type channel block in the presence of 100 (A) and 1,000 (C) μM internal Ba++. A463C channel block in the presence of 100 (B) and 1,000 (D) μM internal Ba++. The data are quantified as percent current versus Ba++ concentration (E). (•) Wild-type data, (○) A463C data. The single binding isotherm fits yield K ds of 75 μM for the wild-type channel and 350 μM for A463C.