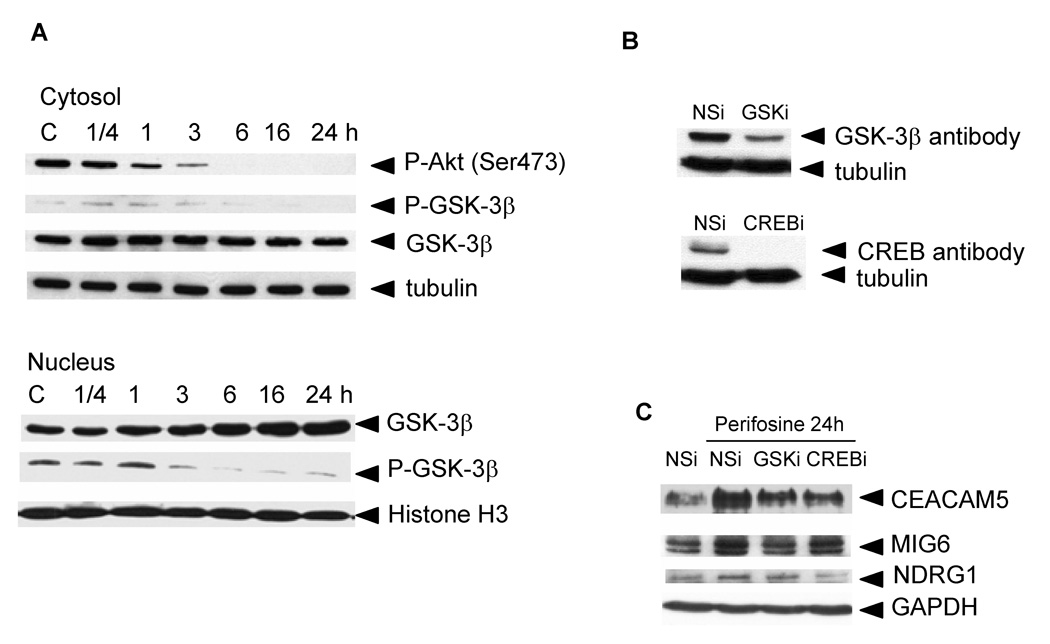
Figure 3. Perifosine-induced Akt inhibition, GSK-3β activation and translocation to the nuclei (A), GSK-3β and CREB protein levels in siRNA-transfected PC-3 cells (B), and GSK-3β and CREB-dependent proteins expression (C).
A, PC-3 cells were treated for indicated periods of time with 5 µM perifosine. Cytosolic and nuclear extracts were prepared as described in Material and Methods. Cytosolic and nuclear proteins were loaded at 5 µg per lane. Western blotting was performed as described in Material and Methods. Membranes were incubated with an anti-P-Akt (Ser473), P-GSK-3β (Ser9), and GSK-3β antibodies. Appropriate secondary antibodies labeled with horse peroxidase were used. Tubulin was used to monitor equal loading of cytosolic proteins and Histone H3 for nuclear proteins. Membranes were developed as described in Materials and Methods. Experiments were repeated three times and representative blots are shown. B, PC-3 cells transfected with non-specific (NSi) or specific siRNA (GSKi, CREBi) duplexes were treated with 5 µM perifosine for 24 h as described in Material and Methods. Cell extracts were prepared to evaluate protein levels of GSK-3β and CREB proteins in transfected cells by western blotting as described in Material and Methods. Experiments were repeated three times with similar results. C, Cell extracts collected at B were used to monitor changes in protein levels of CEACAM5, NDRG1, MIG6, and p21Cip1. GAPDH was used to monitor equal protein loading. Experiments were repeated three times and typical results are presented.