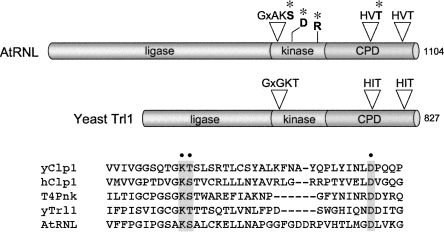
FIGURE 1.
Domain organization of yeast and plant tRNA ligases and conservation of polynucleotide kinase motifs in tRNA repair kinases and yeast and human Clp1. (A) The tRNA ligases of yeast (Trl1) and plant (AtRNL) are composed of three discrete catalytic domains: an N-terminal ligase module; a central 5′-OH polynucleotide kinase module; and a C-terminal RNA 2′,3′ cyclic phosphodiesterase (CPD) module. The positions of the P-loop motif and essential aspartate at the kinase active site and the two HxT motifs that comprise the CPD active site are depicted above the AtRNL polypeptide. (*) Lethal and conditional AtRNL alleles used in the present study contained substitutions for these residues. (B) Alignment of the amino acid sequences in the vicinity of the P-loop of yeast and human Clp1, yeast and plant tRNA ligases, and T4 Pnk. (Gray boxes, dots) The conserved lysine, serine/threonine, and aspartate residues subjected to alanine mutagenesis.