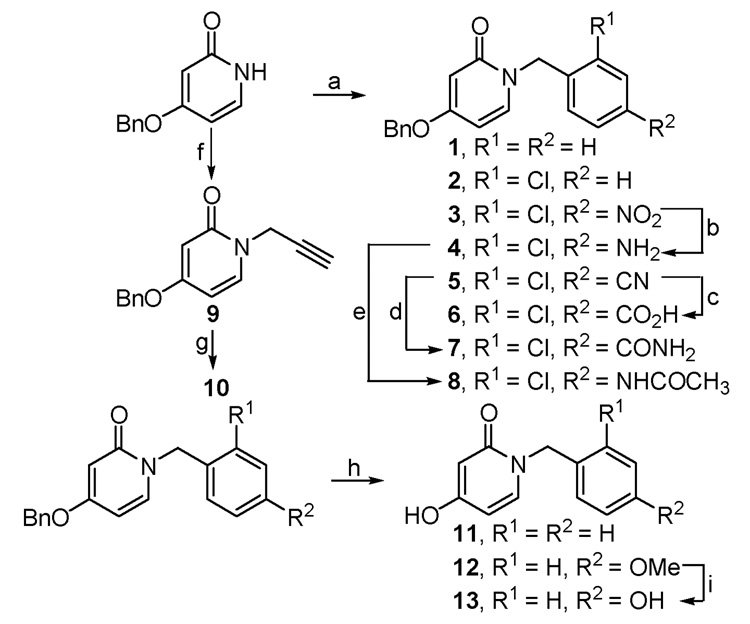
Scheme 1.
Reagents and conditions: (a) KOtBu, TBAI, THF, ArCH2X, 0–25 °C, 16 h; (b) NaBH4, Cu(OAc)2, THF, 0 °C, 40 min; (c) 25% NaOH, EtOH, reflux, 20 h; (d) 35% H2O2, 3N NaOH, EtOH, 30 °C, 20 h; (e) Ac2O, DMAP, Et3N, CH2Cl2, 0–25 °C, 3 h; (f) KOtBu, TBAI, THF, propargyl bromide, 0–25 °C, 16 h; (g) ethyl chlorooximidoacetate, Et3N, rt, 5 h; (h) Pd/C, H2, MeOH, rt, 10–15 min; (i) BBr3, CH2Cl2, −78 °C to rt, 12 h.