Figure 4.
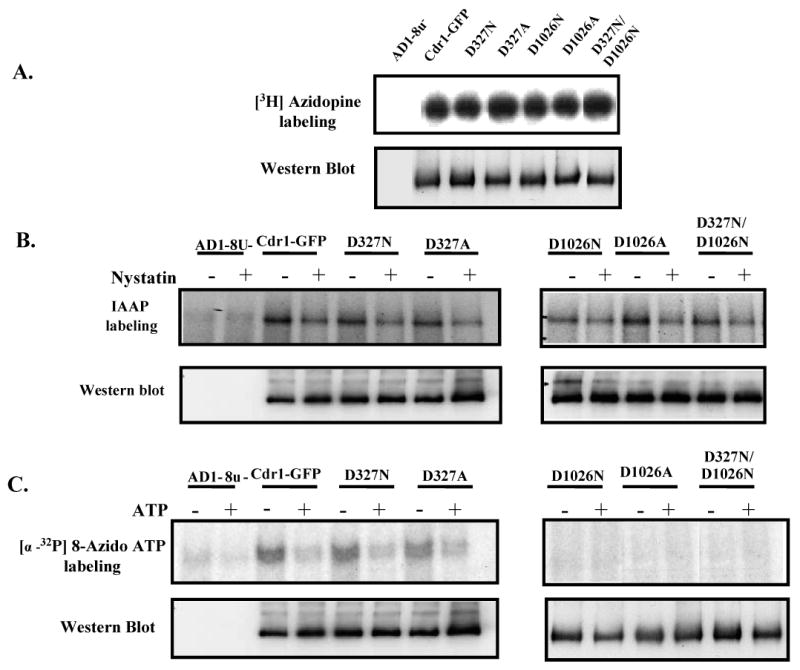
(A) Photoaffinity labeling of wild type Cdr1p and its mutant variants with [3H]-azidopine: The PM fraction (30 μg) of cells expressing the wild type Cdr1p and its mutant variants were incubated with 0.5 μM [3H]-azidopine (60 Ci/mmol) for 5 min under subdued light. The samples were processed and analyzed as described in ‘Experimental Procedures’ and loaded as AD1-8u- (control; lane 1), Cdr1-GFP (lane 2), Cdr1p D327N (lane 3), Cdr1p D327A (lane 4), Cdr1p D1026N (lane 5), Cdr1p D1026A (lane 6) and Cdr1p D327N/D1026N (lane 7). (B) Photoaffinity labeling of wild type Cdr1p and its mutant variants with [125I]-IAAP: The PM fraction (30 μg) of cells expressing the wild type Cdr1p and its mutant variants were incubated with 7.5 nM [125I]-IAAP (2200 Ci/mmol) in presence and absence of 2 μM Nystatin (+ lane) as described in ‘Experimental Procedures’. (C) Photoaffinity labeling of wild type Cdr1p and its mutant variants with [α-32P] 8-azido ATP: The PM fraction (30 μg) of cells expressing the wild type Cdr1p and its mutant variants were incubated with 10 μM [α-32P] 8-azido ATP 7.5 μCi/nmole at 4°C and competed with 10 mM cold ATP (+ ATP lane) as described in ‘Experimental Procedures’. In the Fig. 4A, B and C, lower panel shows the immunoblotting using anti-GFP antibody to ensure an equal loading of wild type Cdr1p and its mutant variants in all the lanes.
