Abstract
Omega-3 (or n-3) polyunsaturated fatty acids (PUFAs) and their metabolites are natural ligands for peroxisome proliferator receptor activator (PPAR)γ and, due to the effects of PPARγ on cell proliferation, survival, and differentiation, are potential anticancer agents. Dietary intake of omega-3 PUFAs has been associated with a reduced risk of certain cancers in human populations and in animal models. In vitro studies have shown that omega-3 PUFAs inhibit cell proliferation and induce apoptosis in cancer cells through various pathways but one of which involves PPARγ activation. The differential activation of PPARγ and PPARγ-regulated genes by specific dietary fatty acids may be central to their distinct roles in cancer. This review summarizes studies relating PUFAs to PPARγ and cancer and offers a new paradigm relating an n-3 PUFA through PPARγ to the expression of the cell surface proteoglycan, syndecan-1, and to the death of cancer cells.
1. INTRODUCTION
The peroxisome proliferator-activated receptor (PPAR) family of nuclear receptors comprises three distinct gene products, PPARα, β/δ, and γ, that differ in ligand specificity, tissue distribution, and developmental expression [1–3]. PPARs demonstrate a relatively high level of constitutive transcriptional activity which is further increased upon binding their activating ligands [4–7]. These ligands are primarily long chain unsaturated and polyunsaturated fatty acids (PUFAs) and certain metabolites of these fatty acids [8–10]. Initially, PPARs were thought mainly to govern lipid homeostasis by binding fatty acids and their metabolites to thereby become more active in regulating genes for proteins involved in lipid metabolism [8, 10, 11]. Indeed, PPARα is expressed predominantly in tissues with high fatty acid requirements such as liver, heart, and kidney, while PPARγ isoforms γ1 and γ2 are highly enriched in adipose tissue to regulate adipocyte differentiation and lipid storage [3]. However, expression of PPARγ1, as with PPARβ/δ and PPARα, has now been extended to most other tissues and regulatory roles for PPARs extended to other systemic functions such as carbohydrate regulation, immune modulation, and the proliferation, survival and differentiation of cells [3]. The latter effects have led to intense interest in the PPARs in relation to cancer.
PPARα and its ligand activators regulate fatty acid and lipoprotein metabolism and promote the development of hepatocellular carcinoma in rodents and reduce the metastasis of melanoma in hamsters [12]. These and other of their effects do not, in general, translate to humans. PPARβ/δ plays a key role in lipid metabolism of peripheral tissues. Its high expression in colon has been shown to promote colon cancer [12, 13], in a mechanism that involves the stimulation of PPARβ/δ by arachidonic acid, PPARβ/δ-dependent upregulation of cyclooxygenase (COX)-2 leading to overproduction of prostaglandin (PG)E2, and PGE2-induced growth of colon cancer cells. There is relatively little documentation of a role for PPARβ/δ in other cancers [14]. By contrast, PPARγ has a broad range of effects on cancer. PPARγ controls fat metabolism by regulating genes involved in lipogenesis, insulin sensitivity, and adipocyte differentiation [3, 15]. These effects underlie the use of thiazolidinediones, which bind and activate PPARγ, to treat insulin-resistant type II diabetes [3, 15]. Although PPARγ activators have been widely shown to inhibit growth in cultured cancer cells, in vivo effects have proved to be complex: they inhibit but sometimes promote cancer growth [16] probably due to stimulation of antiproliferative and apoptotic signaling pathways or proliferative and antiapoptotic pathways, depending on cellular conditions [3, 12, 15–18]. These findings led to the idea of selective PPARγ modulators (SPARMs), drugs analogous to selective estrogen receptor modulators (SERMs) in which distinct actions of the modulator depend on the cellular context [19] and on distinct receptor conformations, and therefore different gene interactions [20]. Fatty acids may be considered as natural SPARMs since their binding does not necessarily lead to PPAR activation and target gene transcription [11].
The considerations discussed above raise a possibility that managed alterations in the type of fatty acids in tissues, can alter the activity of PPARs and thereby the genes they control for therapeutic benefit. The fatty acid content of tissues is dependent mainly on dietary intake. Omega-3 PUFAs, docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) are enriched in the diets of many populations that enjoy a low incidence of cancer [21]. These diets also obtain some modest success ameliorating advanced cancer in humans [22] and have been widely used to inhibit carcinogenesis and tumor progression in animal models. The ability of specific fatty acids to differentially activate PPARs and PPAR-regulated genes may be central to their distinct roles in cancer. This review will focus on PPARγ, its activation by fatty acids, and functional results in cancer cells.
2. FATTY ACID METABOLISM
2.1. Fatty acid types and interconversions
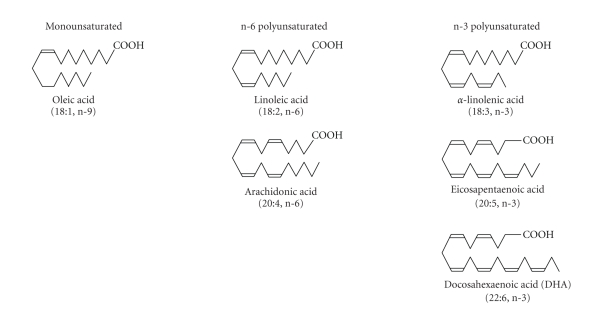
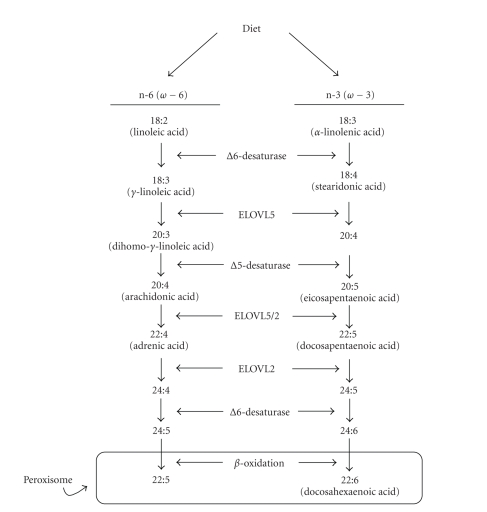
Fatty acids are hydrocarbons with a terminal carboxyl group. The carbons of saturated fatty acids are all connected by single bonds, whereas the chains of monounsaturated and polyunsaturated fatty acids (PUFAs) contain one or more double bonds, respectively. The n-3 and n-6 designation describes the position of the double bond closest to the (omega) carbon at the methyl end of the molecule (Figure 1). Oleic acid (18 : 1) has a single double bond between carbons 9 and 10 from the omega carbon and is designated an n-9 or omega-9 monounsaturated fatty acid. Like the saturated fatty acids, oleic acid can be synthesized de novo in mammalian cells. It can also be obtained from the diet through intake of oils such as olive and canola. By contrast, PUFAs cannot be synthesized de novo in mammals and must be obtained from the diet. The shortest of the n-6 PUFAs is linoleic acid (LA, 18 : 2, n-6). Its 18 carbon, n-3 counterpart is α-linolenic acid (ALA, 18 : 3, n-3). Both LA and ALA are metabolized through a series of elongation and desaturation steps to longer chain PUFAs: LA to arachidonic acid (AA, 20 : 4, n-6) and ALA to EPA (20 : 5, n-3) and DHA (22 : 6, n-3) (Figure 2). The first and rate limiting step in this pathway is the introduction of a double bond by the Δ6 desaturase (for review see [23]). For n-3 PUFAs, ALA is converted to stearidonic acid (SDA, 18 : 4, n-3), elongated, and desaturated by Δ5-desaturase to form EPA. In mammalian cells, the conversion of EPA to DHA follows the Sprecher pathway in which EPA is elongated to docosapentaenoic acid (DPA, 22 : 5, n-3), then to tetracosapentaenoic acid (TPA. 24 : 5, n-3), and desaturated to tetracosahexaenoic acid (THA, 24 : 6, n-3). THA is translocated from the endoplasmic reticulum to peroxisomes, where β-oxidation results in the loss of 2 carbons to form DHA [24]. The PUFAs are also metabolized, most importantly for this review, to PPARγ activators (see Section 2.3).
Figure 1.
Structures of unsaturated fatty acids: oleic acid (n-9 monounsaturated), linoleic acid and arachidonic acid (n-6 polyunsaturated), α-linoleic acid, eicosapentaenoic acid, and docosahexaenoic acid (n-3 polyunsaturated ). The “n” numbers are counted from the methyl or omega terminus.
Figure 2.
The elongation-desaturation pathway for the metabolism of n-6 and n-3 polyunsaturated fatty acids.
2.2. Dietary fatty acids
The results of both dietary intake and stable isotope studies have shown that the conversion of ALA to DHA in humans is extremely inefficient (for review see [25]). Most of the ingested ALA is an immediate target for β-oxidation to provide energy, leaving an estimated 8–10% to enter the elongation-desaturation pathway [26, 27]. A kinetic analysis of 2H-labeled fatty acids estimated that conversion of ALA to EPA was only 0.2%, EPA to DPA was 0.13%, and DPA to DHA was 0.05% [28]. There is some evidence of gender-related differences in the activity of the elongation-desaturation pathway that result in a greater efficiency of conversion of ALA to DHA in females than in males [25, 27, 29]. Support for a role of sex hormones in the conversion pathway is provided by data indicating higher DHA in plasma lipids associated with oral contraceptive use [27] as well in males supplemented with estrogen during sex-change procedures [30]. Moreover, testosterone treatment of female-male transsexuals was shown to decrease plasma DHA [30].
Because common enzymes in the elongation-desaturation pathway are responsible for conversion of both n-3 and n-6 PUFAs, background diet is also a factor in efficiency of conversion. LA is the most abundant fatty acid in the Western diet with consumption in US that is ten-fold that of ALA (reviewed in [31]). Studies have shown that a high intake of LA is associated with a low conversion of ALA to EPA [26]. In spite of limited metabolism of ALA to its long chain derivatives in the stable isotope tracer studies, feeding studies have consistently shown that increased consumption of ALA does result in higher levels of EPA in plasma or cell lipids [31]. However, there was no measurable increase in DHA in these pools. Likewise diets supplemented with EPA do not result in a detectable increase in plasma DHA [32]. Thus, the inefficiency of this pathway does not appear limited to one step but rather extends throughout the pathway. The consensus of a number of studies is that the only way to increase plasma and tissue levels of a specific PUFA is to increase the consumption of that fatty acid. This may be of particular importance in the light of recent in vitro studies on the antitumor effects of DHA.
2.3. PUFAs metabolism to PPARγ activators
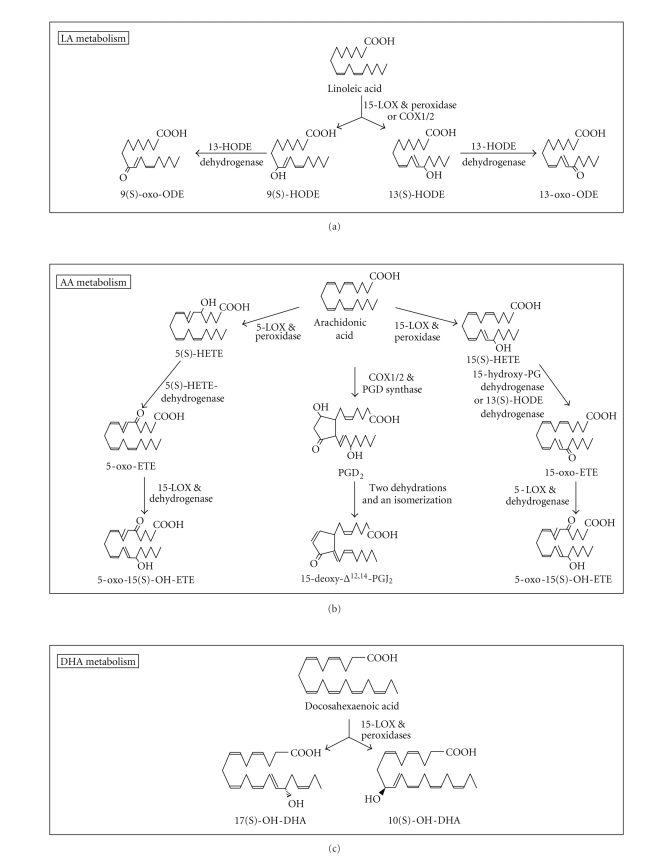
Tissues metabolize PUFAs to oxygenated products that have quite different impacts on PPARγ than their parent molecules. Moreover, n-3 PUFA inhibit the metabolism n-6 PUFAs and subplant them from their oxygenation pathways to form products [33–35]that have different effects on PPARγ than their n-6 PUFAs oxygenated counterparts. It is therefore important to consider PUFAs oxygenation pathways. LA, AA, and DHA require >10–30 μM to activate PPARγ but are commonly converted to stronger (>0.1–10 μM) activators in cells. LA is metabolized (Figure 3, upper panel) by 15-lipoxygenases (LOX)-1/2 to 9(S)- and 13(S)-HODE (hydroxy-octadecaenoate) and by cyclooxygenases (COX)-1/2 to 9(R)- and 13(S)-HODE. The HODEs can be converted to 13-oxo- and 9-oxo-ODE by a dehydrogenase [36–39]. The hydroxy and to a greater extent oxo LA analogs have greater PPARγ-activating potency than LA [36, 40–42]. AA is metabolized (Figure 3, center panel) via 5-LOX to 5(S)-HETE (hydroxy-eicosatetra-enoate) and via 15-LOX-1/2 to 15(S)-HETE. These HETEs can be converted to oxo-ETEs and 5-oxo-15(S)-hydroxy-ETE as shown in Figure 3 [39, 43–50]. 15-HETE has weak and 5-HETE essentially no ability to activate PPARγ. However, their oxo counterparts have appreciable ability to do so with 5-oxo-15(S)-hydroxy-ETE showing the greatest potency in binding and activating PPARγ [43]. AA is also metabolized (Figure 3, center panel) by COX1/2 to PG (prostaglandin) D2 which as a consequence of successive dehydrations and an isomerization, perhaps by nonenzymatic routes, convert to PGJ2, Δ12-PGJ2, and 15-deoxyΔ12,14-PGJ2(15-d-Δ12,14-PGJ2); these PGJ2's have greater ability than PGD2 to activate PPARγ with 15-d-Δ12,14-PGJ2 being a most potent (>0.1–1 μM) naturally occurring PPARγ activator [9, 43, 51–56]. In one study, the Kd's of 15-d-Δ12,14-PGJ2, 5-oxo-15-OH-ETE, PGJ2, 5-oxo-ETE, and 5(S)-HETE in binding to PPARγ were 1.4, 11, 37, 81, and >1000 μM, respectively; their potency in activating a cell-based PPARγ reporter paralleled these Kd's [43]. DHA is metabolized (Figure 3, bottom panel) by 15-LOX or other oxygenase to 17-OH- and 7-OH-DHA, products that activate PPARγ with greater potency (ED50's in activating a cell-based PPARγ reporter of ~5 μM) than DHA [57]. 4-OH-, and 4-oxo-DHA [53], while not yet shown to be made by cancer cells, also activate PPARγ with greater potency (ED50's of 13.4 and 7.8 μM in activating a cellular PPARγ reporter, resp.) than DHA (ED50 > 10 μm) [53]. Hence, in this DHA series, similar to the 5-HETE series of AA metabolites, the oxo analog exhibits the greatest potency. We note that the more potent PPARγ activators, the oxo-PUFAs, form preferentially in cells undergoing excessive oxidation, free radical, and NADPH/NADH-depleting reactions [43, 44, 48, 57, 58]. This suggests that PPARγ may serve as a sensor for oxo-PUFA thereby monitoring cellular oxidative stress and when this stress is severe, engaging cell death programs [43, 58]. This PPARγ function, we suggest, could contribute to the necrosis that occurs in tumors particularly after chemical and radiation treatment [59].
Figure 3.
The cellular metabolism of LA, AA, and DHA to more potent activators of PPARγ. ODE is octadecaenoate; HETE is hydroxy-eicosatetraenoate; ETE is eicosatetraenoate; PG is prostaglandin.
Cells process PUFAs in other relevant ways. They convert them to nitrates, probably in nonenzymatic reactions, where the nitric oxide made during cell stimulation attacks the PUFAs. Nitrated LA and AA are stronger PPARγ activators than their precursors [60–62]. Cells also convert PUFAs to cannabinoids such as anandamide (ethanolamine amide of AA) and arachidonoylglycerol which also activate PPARγ with greater potency than AA [63–65]. Finally, cells conjugate glutathione to PUFAs that contain an α,β-unsaturated ketone such as 15-d-Δ12,14-PGJ2 and 5-oxo-ETE [66–68]. Since the conjugates are rapidly excreted from cells by multidrug-resistance transporters, conjugation inhibits the ability of α,β-unsaturated ketones to activate PPARγ [66]. Cancer cells excrete anticancer drugs through these same transporters and become drug-resistant by overexpressing these transporters [69]. Such mutated cells may also be resistant to α,β-unsaturated ketone activators of PPARγ.
2.4. Low-density lipoproteins (LDL) as deliverers of PPARγ-activating n-3 PUFA
LDL carry esterified PUFAs in glycerolipids and cholesterol. They bind to cell surface LDL receptors and then internalize in endocytic vesicles which merge with lysosomes to de-esterify and release the PUFAs into the cytosol [70]. This route differs from the direct delivery of PUFA: it bypasses cell surface G protein-coupled fatty acid receptors (GPR 40 and 120; see Section 4.3), deposits PUFA in cells more slowly, and thereby avoids stimulation of G protein-coupled receptors and, perhaps, an array of C domain-bearing proteins which are activated by PUFA. This is also an important pathway for delivering PUFA to tumor cells because of a significant increase in LDL receptor activity in neoplastic tissues [71–73]. We have obtained from monkeys fed special diets, LDL enriched with n-6 PUFA (mostly AA and LA) or n-3 PUFA (mostly DHA and EPA). The n-3 but not n-6 PUFA-rich LDL mimicked thiazolidinediones and DHA in inhibiting cancer cell growth [74] and activating PPARγ [75, 76].
3. PPARγ
3.1. Structural considerations
PPARγ1 and γ2 originate from the PPARγ gene through separate promoters and 5′ exons. Compared to the ubiquitously expressed PPARγ1, PPARγ2, which is limited mainly to adipose tissue, has 30 additional amino acids at its NH2 terminus and is a more potent transcription activator [77]. Because they appear to have the same targets, however, the two isoforms are here considered together under the term PPARγ. PPARγ is comprised of four functional parts: the NH2-terminal A/B region bears a ligand-independent transcription-activating motif AF-1; C region binds response elements (PPREs with a DR-1 consensus half-sequence of AGGTCA); D region binds various transcription cofactors; and E/F region has an interface for dimerizing with 9-cis retinoic acid receptors (RXRs), an AF-2 ligand-dependent transcription-activating motif, and a ligand-binding domain (LBD) [3, 12, 15, 17]. The LBD has a spacious cavity that binds ligands having a polar head group extending from a hydrophobic tail such as diverse PUFAs and PUFA metabolites [7, 77].
3.2. PPARγ regulation by other signaling pathways
PPARγ is phosphorylated by extracellular signal-regulated kinases (ERK)-1/2 and C-Jun N-terminal kinase; when so phosphorylated, it has less ligand-binding affinity and gene-regulating activity [3, 78, 79]. The phosphorylation and attendant decrease in activity of PPARγ occur in cells treated with PPARγ activators and may cause the activators to show little or no ability to stimulate PPARγ [3, 79–81]. ERK pathways impact PPARγ in another way: the ERK-activating enzyme, MEK, when activated, binds with PPARγ's AF-2 motif. This causes PPARγ to release from PPRE complexes and, bound to MEK and directed by MEK's nuclear export signal, to exit the nucleus [81, 82]. It is important to note that PUFAs and PUFA metabolites can activate the MEK/ERK pathway (see Section 4.3) and therefore may have biphasic effects: they not only directly activate PPARγ but also entrain events inhibiting PPARγ.
PPARγ is targeted for degradation by ubiquitylation and sumoylation. Ligand binding, certain protein kinases, and some transcription cofactors (e.g., p300) promote ubiquitin-dependent degradation of PPARγ in proteasomes [3]. Sumoylation occurs on K107 of PPARγ2 in a ligand-independent fashion to inhibit AF-1 function and on K365 of PPARγ in a ligand-dependent fashion to promote PPARγ's binding of nuclear receptor corepressor [83, 84]. Sumoylation of PPARγ causes its proteasomal degradation. ERK phosphorylation promotes K107 sumoylation. This reaction represents yet another means by which ERKs can inhibit PPARγ [84].
3.3. PPARγ transcriptional cofactors
PPARs bind a specific DNA sequence termed peroxisome proliferator response element (PPRE) in the 5′-flanking region of target genes as a heterodimer with RXR. Studies using various techniques [3, 85, 86] suggest the following model: PPARγ•RXR complexes (the interaction is ligand-independent) exist in nuclei as macrocomplexes associated with various transcription corepressors [3, 87]. Some complexes, ligand-bound or not, may associate with transcription coactivators to control the basal expression of genes. In any event, PPARγ•RXR complexes are highly mobile, rapidly scanning chromatin, although this scanning does not involve their DNA binding domain [86]. Ligands trigger PPARγ•RXR to localize at their cognate PPREs and to exchange corepressors for coactivators such as cyclic AMP response element binding protein (CREB) and p300 [3, 16, 87, 88]. At some gene sites, activators cause PPARγ•RXR to recruit corepressors and thereby cause gene repression [3, 89, 90]. However, the availability of cofactors differs between cell types and within cells over time depending on the cell's history and the association of the cofactors to other genes [3, 15, 16], for example, activation of PPARγ deprives T cell factor/lymphoid enhancing factor (TCF/LEF) of cofactors to thereby inhibit oncogenic signaling by the Wnt pathway [16]. Thus, the effects of PPARγ activation vary depending on context and cofactor availability at each genetic site. It seems at least possible that the PUFA ligands for PPARγ will have differential effects in impacting its interactions with these transcriptional cofactors in a manner similar to the SPARMs model [19].
4. TARGETS OF PPARγ RELEVANT TO CANCER
4.1. Gene targets of PPARγ
Most known target genes of PPARγ regulate lipid metabolism and transport [15]with few cancer-related genes having been confirmed as induced by PPARγ. PPARγ does induce G0/G1 switch gene 2 whose product causes growth arrest in 3T3-L1 cells [91, 92]. PPARγ also binds the NFκB promoter of p53 to stimulate expression of p53 and, in consequence, p21WAF1/Cip1. It also binds to a promoter in the Fas ligand gene to induce the expression of this member of the extrinsic apoptosis pathway. These effects appear responsible for slowing growth and causing apoptosis in MCF7 breast cancer [93], human umbilical vein endothelial [94], and possibly Reh [95] cells. Recent studies have identified the heparan sulfate proteoglycan, syndecan 1, as a target for PPARγ in human breast [75, 76] and prostate [96] cancer cells. The upregulation of syndecan 1 by PPARγ resulted in apoptosis induction [76].
4.2. Other targets of PPARγ
PPARγ impacts many growth-promoting elements through its secondary actions that, while ligand-dependent, do not directly involve its gene promoters. It interacts with nuclear factor of activated T cells, phosphorylated signal transducer, and activator of transcription (STAT)-3, and nuclear factor κB (NFκB) to block signaling through these pathways [3]. It binds transcription cofactors to alter these cofactors' availability to other transcription factors: ligand bound-PPARγ deprives NFκB of AP-1; deprives STAT-1 of CREB binding protein; and releases SMRT to render it available to repress STAT-3's transcriptional activity [3, 16, 17, 97]. PPARγ activation is also associated with the activation of ERK1/2, protein kinases C, protein kinase A, AMP-activated protein kinase α [17]; induction of p16, p18, and p21 cyclin-dependent kinase inhibitors [3, 17, 18]; decreased expression of cyclooxygenase 2, cmyc, cmyb, D1, and D3 cell cycle control genes, and regenerating gene 1A [17, 18]; decreased secretion of cytokines and growth factors [17, 98]; depression of the Akt survival pathway by upregulating PTEN and inhibiting the phosphorylation of Akt and mTOR [3, 17]; inhibiting retinoblastoma protein (Rb) activity to repress the activities of cyclins D3 and E [3]; and regulating a host of other elements involved in the growth and death of cells [3, 12, 16–18]. It is not clear which if any of these effects are due to the action of PPARγ or PPARγ activators. PUFAs impact many of these same targets but can do so not only by PPARγ-dependent but also PPARγ-independent routes (see the next section).
4.3. Targets of PPARγ-activating Ligands
Studies of PPARγ function depend on challenging cells with PPAR-activating ligands that have numerous side effects impacting cell growth. 15-d-Δ12,14-PGJ2 has a reactive α,β-unsaturated ketone (Figure 3) that covalently binds to cysteine sulfur on PPARγ; this renders its PPARγ binding irreversible [58, 68]. 15-d-Δ1,14-d-PGJ2 also binds to cysteines in the IKKβ subunit of IκB kinase, thereby inhibiting NFκB activation [99, 100]. Other ligands with an α,β unsaturated ketone (e.g., oxo-ODEs and oxo-ETEs; see Figure 3) have this chemical reactivity [58] and along with 15-d-Δ1,14-d-PGJ2 may exert anticancer effects by covalently attaching to signal molecules like IKKβ [58, 99, 101] or elements needed for expressing the epidermal growth factor receptor (EGFR) and JAK [102, 103].
Naturally occurring ligands have other PPARγ-independent effects. The D and J series of PGs including 15-d-Δ12,14-PGJ2 bind to PGD2 receptors [104], 5-oxo- and 5-oxo-15-hydroxy-ETE bind to the OXE receptor [105], and AA, EPA, and DHA bind to GPR40 and GPR120 receptors [106, 107]. These G protein-coupled receptors regulate signal pathways that effect cancer cell growth. For example, 5-oxo-15-hydroxy ETE acts on OXE to stimulate cells to activate ERK and Akt and proliferate; this stimulation counters its antigrowth activity in various cancer cell types. Indeed, HEK293 cells lack OXE receptors and in contrast to OXE receptor-bearing breast, prostate and ovarian cancer cell lines respond to 5-oxo-ETE and 5-oxo-15-oxo-ETE only by slowing, not speeding, their proliferation [43]. PUFAs activation of GPR120 also causes ERK and Akt activation to increase the survival of serum-starved STC-1 cells [108]. Finally, PUFAs are also metabolized to products that act on G protein receptors to promote cell growth, for example, prostate cancer cells convert AA to PGE2, which acts through its receptors to stimulate the NFκB pathway and thereby the expression of various cytokines and growth factors [109]. The G protein receptor-dependent actions of PPARγ ligands may explain reports that these ligands have biphasic effects in stimulating proliferation and antiproliferation in cancer cells [110].
Thiazolidinediones stimulate cells to activate ERK1/2, p38, and JNK [111–113] by discharging Ca2+ from the ER to evoke an ER stress response; this activates Ca2+/calmodulin kinase II, proline-rich tyrosine kinase 2, protein kinases C, c-Src, EGFR, the ERK1/2 and JNK pathways, the double stranded RNA-activated protein kinase, and p38 [111]. Double stranded RNA-activated protein kinase inactivates eukaryotic initiation factor-2 to depress protein translation [111, 114]. Since EPA has recently been shown to have similar effects on ER calcium discharge [111, 115], it seems likely that various other PUFAs activate the ER stress pathway. Nonetheless, PPARγ activators often show very different side effects [42, 103, 116–120]. For example, among three PPARγ agonists, ciglitazone, 9-HODE, and 13-HODE, only 9-HODE induced apoptosis in U937 cells [38], 15d-Δ12,14-PGJ2, but not various other PPARγ ligands, reduced EGFR expression in squamous carcinoma cells [99], 15d-Δ12,14-PGJ2, but not troglitazone, inhibited the stimulated induction of MHC class II molecules in retinal pigmented epithelial cells [112], and DHA, but not EPA, stimulated the target gene, syndecan 1 to inhibit the proliferation and induce apoptosis in breast and prostate cancer cell lines [75, 76, 96]. Numerous other examples of differential effects among PPARγ agonists exist (e.g., [113–116]), but it is worth stressing that n-3 PUFAs inhibit the metabolism of n-6 PUFAs to products that promote the growth of cancer cells such as PGE2, 5-HETE, and leukotriene B4 [33–35, 45, 113]. This inhibitory effect may make an important contribution to the anticancer effects of n-3 PUFAs.
5. DIETARY FATTY ACIDS AND CANCER
5.1. Human studies
Although there are inconsistencies [121], human population studies have shown that consumption of a diet enriched in n-3 PUFAs may offer protection against a number of cancers including those of breast [122–124], prostate [125, 126], and colon [127–129]. Although many of these studies have relied on dietary intake data from self-reported questionnaires or estimates based on national consumption, a few have used the fatty acid composition of tissues as a measure of exposure to dietary fats. The EURAMIC study is one of the largest to provide evidence that the balance between n-3 and n-6 PUFA may play a role in breast cancer [130]. Adipose tissue aspirates from breast cancer patients and controls demonstrated that the ratio of long chain n-3 to n-6 PUFAs was inversely associated with breast cancer in four of five centers studied. In human prostate tissue, lower EPA and DHA as well as lower n-3 to n-6 PUFAs ratios were associated with cancer compared to benign prostate hyperplasia [131] and with advanced stage compared to organ confined disease [132]. This inverse association of n-3 PUFAs and prostate cancer is supported by analyses of fatty acids in serum and red-cell membranes of patients with prostate disease [133, 134].
5.2. Animal studies
Animal studies provide convincing evidence of a negative relationship with n-3 PUFA diets and a positive relationship with n-6 PUFA diets for breast, prostate, and colon cancer. In studies of breast cancer induced by chemical carcinogens in rats [135–137], and human cancer cell xenografts in nude mice [138–140], tumor growth rate, size, and metastases were all suppressed by dietary n-3 PUFA supplementation. Likewise for colon cancer, antitumor properties of n-3 PUFA diets have been shown in transplantable mouse tumors [141–143] as well as in chemically induced rat tumors [144–151]. Although there have been fewer animal studies of PUFAs in prostate cancer, they are consistent with those in breast and colon cancer. In xenograft models of prostate cancer, n-3 PUFAs enriched diets inhibited tumor growth compared to n-6 PUFA diets [152–154]. Recently, a prostate-specific Pten knockout mouse model was used to demonstrate that a dietary ratio of n-6 to n-3 PUFA lower than 5 was effective in suppressing tumor growth, and extending animal lifespan [155].
5.3. Cell culture studies
Insight into the mechanism(s) responsible for the anticancer properties of n-3 PUFAs have been provided by animal studies as well as by in vitro investigations using human cancer cell lines. A major focus for such studies has been the competitive inhibition between n-6 and n-3 PUFAs for the enzymes involved in their metabolism. The desaturation and elongation of LA to AA were shown to be decreased in the presence of high n-3 PUFAs due to enzyme preference for the n-3 substrates [156]. AA and EPA compete for the COX and LOX enzymes, again with preferential n-3 utilization that results in a reduction in the highly reactive eicosanoids generated from AA [157, 158] in favor of less inflammatory n-3 eicosanoids [159]. The decreased growth of prostate xenograft tumors was shown to involve inhibition of COX 2 and PGE2 in the tissues [154]. Thus, the combined human, animal, and cell culture studies indicate that diet is an important regulator of the levels of n-3 versus n-6 PUFAs in tissues, including those that are cancerous. High levels of n-3 PUFAs may directly evoke antitumor events, become metabolized to products with antitumor activity, or suppress the production of tumor-promoting metabolites such as those formed by n-6 PUFAs.
6. n-3 PUFA REGULATION OF SYNDECAN-1
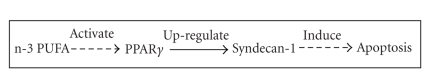
Increasing evidence implicates PPARγ in the divergent effects of n-3 and n-6 PUFAs in cancer cells and point to a growth inhibitory role for PPARγ [160–164]. We recently found that n-3 PUFAs—but not n-6 PUFAs—enriched LDL, inhibited the proliferation, and induced apoptosis in human breast cancer cells [74–76]. The n-3 LDL delivered both EPA and DHA to the cells. When these individual fatty acids were delivered to cells by albumin, DHA but not EPA proved effective in stimulating apoptosis in a pathway that involved activation of PPARγ [75]. The molecular target for both DHA and PPARγ in these cells was shown to be the heparan sulfate proteoglycan, syndecan-1. Syndecan-1 itself was effective in apoptosis induction and when syndecan-1 was silenced, the ability of DHA to induce apoptosis was completely blocked as it was in the presence of a dominant negative PPARγ [76]. Moreover, syndecan-1 siRNA was effective in blocking troglitizone-induced apoptosis. Thus, a novel pathway linking n-3 PUFAs to apoptosis in tumor cells is as follows: DHA activates PPARγ, which results in transcriptional upregulation of the syndecan-1 target gene, and the syndecan-1 protein induces apoptosis (Figure 4). This novel pathway has been confirmed in human prostate cancer cells [96].
Figure 4.
The syndecan-1 pathway for n-3 PUFA induction of apoptosis. Dashed lines indicate that effects may be indirect with involvement of other metabolites and signaling molecules.
Although PPARγ was not a target for EPA in breast and prostate cancer cells, a recent report has demonstrated that EPA was an effective PPARγ transactivator in HT-29 human colon cancer cells [165]. In contrast, both EPA and DHA were shown to reduce PPRE reporter activity in an HCT-116 colon cancer cells [166]. DHA has recently been shown to reduce the growth of human lung cancer cells in a process that was associated with increased PPARγ protein [167]. These conflicting reports are consistent with data showing selective modulation of PPARγ by different ligands in different cells [168]. Several other reasons may be proposed for the differential response to DHA and EPA in the breast and prostate tumor cells including (1) PPARγ activation may be mediated by a unique DHA metabolite rather than DHA itself; (2) there may be a difference in the bioavailability of the two fatty acids following cellular uptake; (3) EPA may be a ligand for or metabolized to a ligand (e.g., 5(S)-hydroxy-eicosapentaenoic acid) for a G protein-coupled receptor that activates ERK and thereby inactivates or in some other way counteracts PPARγ; (4) EPA may directly, or after being metabolized, activate other pathways that counteract PPARγ signaling.
The identification of syndecan-1 as a target gene for PPARγ in the breast and prostate cancer cells was a novel but not unexpected finding. The syndecan-1 promoter contains a DR-1 element that is recognized by a several members of the nuclear hormone receptor superfamily including PPARγ. Although there are conflicting reports of a role for syndecan-1 in cancer, the importance of these studies is the identification of a PPARγ molecular target that is regulated by PUFAs and results in functional response in the tumor cells. As more such targets emerge, we may be able to understand how different dietary fatty acids play divergent roles in cancer.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants P01CA106742 (IJE, JO) and R01CA115958 (IJE). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Kersten S, Desvergne B, Wahli W. Roles of PPARS in health and disease. Nature. 2000;405(6785):421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 2.Lee C-H, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144(6):2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 3.Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Progress in Lipid Research. 2006;45(2):120–159. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Lazennec G, Canaple L, Saugy D, Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Molecular Endocrinology. 2000;14(12):1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molnár F, Matilainen M, Carlberg C. Structural determinants of the agonist-independent association of human peroxisome proliferator-activated receptors with coactivators. The Journal of Biological Chemistry. 2005;280(28):26543–26556. doi: 10.1074/jbc.M502463200. [DOI] [PubMed] [Google Scholar]
- 6.Michalik L, Zoete V, Krey G, et al. Combined simulation and mutagenesis analyses reveal the involvement of key residues for peroxisome proliferator-activated receptor α helix 12 dynamic behavior. The Journal of Biological Chemistry. 2007;282(13):9666–9677. doi: 10.1074/jbc.M610523200. [DOI] [PubMed] [Google Scholar]
- 7.Zoete V, Grosdidier A, Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochimica et Biophysica Acta. 2007;1771(8):915–925. doi: 10.1016/j.bbalip.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Xu HE, Lambert MH, Montana VG, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Molecular Cell. 1999;3(3):397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 9.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ . Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 10.Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ . Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ . Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sertznig P, Seifert M, Tilgen W, Reichrath J. Present concepts and future outlook: function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer. Journal of Cellular Physiology. 2007;212(1):1–12. doi: 10.1002/jcp.20998. [DOI] [PubMed] [Google Scholar]
- 13.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nature Reviews Cancer. 2004;4(1):61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 14.Xu L, Han C, Wu T. A novel positive feedback loop between peroxisome proliferator-activated receptor-δ and prostaglandin E2 signaling pathways for human cholangiocarcinoma cell growth. The Journal of Biological Chemistry. 2006;281(45):33982–33996. doi: 10.1074/jbc.M600135200. [DOI] [PubMed] [Google Scholar]
- 15.Michalik L, Auwerx J, Berger JP, et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacological Reviews. 2006;58(4):726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan A, Nair SA, Pillai MR. Biology of PPARγ in cancer: a critical review on existing lacunae. Current Molecular Medicine. 2007;7(6):532–540. doi: 10.2174/156652407781695765. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Roman J. Peroxisome proliferator-activated receptor γ: a novel target for cancer therapeutics? Anti-Cancer Drugs. 2007;18(3):237–244. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- 18.Wang T, Xu J, Yu X, Yang R, Han ZC. Peroxisome proliferator-activated receptor γ in malignant diseases. Critical Reviews in Oncology/Hematology. 2006;58(1):1–14. doi: 10.1016/j.critrevonc.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Sporn MB, Suh N, Mangelsdorf DJ. Prospects for prevention and treatment of cancer with selective PPARγ modulators (SPARMs) Trends in Molecular Medicine. 2001;7(9):395–400. doi: 10.1016/s1471-4914(01)02100-1. [DOI] [PubMed] [Google Scholar]
- 20.Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. The Journal of Clinical Investigation. 2000;106(4):467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqui RA, Shaikh SR, Sech LA, Yount HR, Stillwell W, Zaloga GP. Omega 3-fatty acids: health benefits and cellular mechanisms of action. Mini Reviews in Medicinal Chemistry. 2004;4(8):859–871. doi: 10.2174/1389557043403431. [DOI] [PubMed] [Google Scholar]
- 22.Colomer R, Moreno-Nogueira JM, García-Luna PP, et al. n-3 fatty acids, cancer and cachexia: a systematic review of the literature. British Journal of Nutrition. 2007;97(5):823–831. doi: 10.1017/S000711450765795X. [DOI] [PubMed] [Google Scholar]
- 23.Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochimica et Biophysica Acta. 2000;1486(2-3):219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 24.Sprecher H. The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins Leukotrienes and Essential Fatty Acids. 2002;67(2-3):79–83. doi: 10.1054/plef.2002.0402. [DOI] [PubMed] [Google Scholar]
- 25.Williams CM, Burdge G. Long-chain n-3 PUFA: plant v. marine sources. Proceedings of the Nutrition Society. 2006;65(1):42–50. doi: 10.1079/pns2005473. [DOI] [PubMed] [Google Scholar]
- 26.Emken EA, Adlof RO, Gulley RM. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochimica et Biophysica Acta. 1994;1213(3):277–288. doi: 10.1016/0005-2760(94)00054-9. [DOI] [PubMed] [Google Scholar]
- 27.Burdge GC, Wootton SA. Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. British Journal of Nutrition. 2002;88(4):411–420. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 28.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of α-linolenic acid metabolism in adult humans. Journal of Lipid Research. 2001;42(8):1257–1265. [PubMed] [Google Scholar]
- 29.Pawlosky RJ, Hibbeln JR, Lin Y, et al. Effects of beef- and fish-based diets on the kinetics of n-3 fatty acid metabolism in human subjects. The American Journal of Clinical Nutrition. 2003;77(3):565–572. doi: 10.1093/ajcn/77.3.565. [DOI] [PubMed] [Google Scholar]
- 30.Giltay EJ, Gooren LJG, Toorians AWFT, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. The American Journal of Clinical Nutrition. 2004;80(5):1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- 31.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. The American Journal of Clinical Nutrition. 2006;83(6):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 32.Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. Journal of Lipid Research. 2003;44(3):455–463. doi: 10.1194/jlr.M200282-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Payan DG, Wong MYS, Chernov-Rogan T, et al. Alterations in human leukocyte function induced by ingestion of eicosapentaenoic acid. Journal of Clinical Immunology. 1986;6(5):402–410. doi: 10.1007/BF00915380. [DOI] [PubMed] [Google Scholar]
- 34.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. The American Journal of Clinical Nutrition. 2000;71(1):343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 35.Gibson RA, Neumann MA, James MJ, Hawkes JS, Hall C, Cleland LG. Effect of n-3 and n-6 dietary fats on the lipoxygenase products from stimulated rat neutrophils. Prostaglandins Leukotrienes and Essential Fatty Acids. 1992;46(2):87–91. doi: 10.1016/0952-3278(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 36.Altmann R, Hausmann M, Spöttl T, et al. 13-oxo-ODE is an endogenous ligand for PPARγ in human colonic epithelial cells. Biochemical Pharmacology. 2007;74(4):612–622. doi: 10.1016/j.bcp.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Earles SM, Bronstein JC, Winner DL, Bull AW. Metabolism of oxidized linoleic acid: characterization of 13-hydroxyoctadecadienoic acid dehydrogenase activity from rat colonic tissue. Biochimica et Biophysica Acta. 1991;1081(2):174–180. doi: 10.1016/0005-2760(91)90023-b. [DOI] [PubMed] [Google Scholar]
- 38.Hamberg M. Stereochemistry of oxygenation of linoleic acid catalyzed by prostaglandin-endoperoxide H synthase-2. Archives of Biochemistry and Biophysics. 1998;349(2):376–380. doi: 10.1006/abbi.1997.0443. [DOI] [PubMed] [Google Scholar]
- 39.Brash AR, Jisaka M, Boeglin WE, et al. Investigation of a second 15S-lipoxygenase in humans and its expression in epithelial tissues. Advances in Experimental Medicine and Biology. 2000;469:83–89. doi: 10.1007/978-1-4615-4793-8_13. [DOI] [PubMed] [Google Scholar]
- 40.Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400(6742):378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 41.Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ . Cell. 1998;93(2):229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 42.Hampel JKA, Brownrigg LM, Vignarajah D, et al. Differential modulation of cell cycle, apoptosis and PPARγ2 gene expression by PPARγ agonists ciglitazone and 9-hydroxyoctadecadienoic acid in monocytic cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 2006;74(5):283–293. doi: 10.1016/j.plefa.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 43.O'Flaherty JT, Rogers LC, Paumi CM, et al. 5-oxo-ETE analogs and the proliferation of cancer cells. Biochimica et Biophysica Acta. 2005;1736(3):228–236. doi: 10.1016/j.bbalip.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Erlemann K-R, Rokach J, Powell WS. Oxidative stress stimulates the synthesis of the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid by inflammatory cells. The Journal of Biological Chemistry. 2004;279(39):40376–40384. doi: 10.1074/jbc.M401294200. [DOI] [PubMed] [Google Scholar]
- 45.O'Flaherty JT, Cordes JF, Lee SL, Samuel M, Thomas MJ. Chemical and biological characterization of oxo-eicosatetraenoic acids. Biochimica et Biophysica Acta. 1994;1201(3):505–515. doi: 10.1016/0304-4165(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 46.O'Flaherty JT, Kuroki M, Nixon AB, et al. 5-oxo-eicosanoids and hematopoietic cytokines cooperate in stimulating neutrophil function and the mitogen-activated protein kinase pathway. The Journal of Biological Chemistry. 1996;271(30):17821–17828. doi: 10.1074/jbc.271.30.17821. [DOI] [PubMed] [Google Scholar]
- 47.Powell WS, Gravelle F, Gravel S. Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)- hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes. The Journal of Biological Chemistry. 1992;267(27):19233–19241. [PubMed] [Google Scholar]
- 48.Powell WS, Gravelle F, Gravel S. Phorbol myristate acetate stimulates the formation of 5-oxo-6,8,11,14- eicosatetraenoic acid by human neutrophils by activating NADPH oxidase. The Journal of Biological Chemistry. 1994;269(41):25373–25380. [PubMed] [Google Scholar]
- 49.Lee SH, Rangiah K, Williams MV, Wehr AY, DuBois RN, Blair IA. Cyclooxygenase-2-mediated metabolism of arachidonic acid to 15-oxo-eicosatetraenoic acid by rat intestinal epithelial cells. Chemical Research in Toxicology. 2007;20(11):1665–1675. doi: 10.1021/tx700130p. [DOI] [PubMed] [Google Scholar]
- 50.Gulliksson M, Brunnström Å, Johannesson M, et al. Expression of 15-lipoxygenase type-1 in human mast cells. Biochimica et Biophysica Acta. 2007;1771(9):1156–1165. doi: 10.1016/j.bbalip.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 52.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in PPARγ associated with human colon cancer. Molecular Cell. 1999;3(6):799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 53.Itoh T, Yamamoto K. Peroxisome proliferator activated receptor γ and oxidized docosahexaenoic acids as new class of ligand. Naunyn-Schmiedeberg's Archives of Pharmacology. 2008;377(4–6):541–547. doi: 10.1007/s00210-007-0251-x. [DOI] [PubMed] [Google Scholar]
- 54.Nosjean O, Boutin JA. Natural ligands of PPARγ: are prostaglandin J2 derivatives really playing the part? Cellular Signalling. 2002;14(7):573–583. doi: 10.1016/s0898-6568(01)00281-9. [DOI] [PubMed] [Google Scholar]
- 55.Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-γ is a new therapeutic target in sepsis and inflammation. Shock. 2005;23(5):393–399. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]
- 56.Scher JU, Pillinger MH. 15d-PGJ2: the anti-inflammatory prostaglandin? Clinical Immunology. 2005;114(2):100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 57.González-Périz A, Planagumà A, Gronert K, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17 S-hydroxy-DHA. The FASEB Journal. 2006;20(14):2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- 58.Shiraki T, Kamiya N, Shiki S, Kodama TS, Kakizuka A, Jingami H. α,β-unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor γ . The Journal of Biological Chemistry. 2005;280(14):14145–14153. doi: 10.1074/jbc.M500901200. [DOI] [PubMed] [Google Scholar]
- 59.Rigas B, Sun Y. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. British Journal of Cancer. 2008;98(7):1157–1160. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freeman BA, Baker PRS, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. The Journal of Biological Chemistry. 2008;283(23):15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker PRS, Lin Y, Schopfer FJ, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. The Journal of Biological Chemistry. 2005;280(51):42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schopfer FJ, Lin Y, Baker PRS, et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor γ ligand. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(7):2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. British Journal of Pharmacology. 2007;152(5):576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenman A, Fowler CJ. Interaction of ligands for the peroxisome proliferator-activated receptor γ with the endocannabinoid system. British Journal of Pharmacology. 2007;151(8):1343–1351. doi: 10.1038/sj.bjp.0707352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARγ transcriptional activation and 3T3-L1 preadipocyte differentiation. European Journal of Pharmacology. 2005;517(3):174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 66.Paumi CM, Smitherman PK, Townsend AJ, Morrow CS. Glutathione S-transferases (GSTs) inhibit transcriptional activation by the peroxisomal proliferator-activated receptor γ (PPARγ) ligand, 15-deoxy-Δ12,14-prostaglandin J2 (15-d-PGJ2) Biochemistry. 2004;43(8):2345–2352. doi: 10.1021/bi035936+. [DOI] [PubMed] [Google Scholar]
- 67.Paumi CM, Wright M, Townsend AJ, Morrow CS. Multidrug resistance protein (MRP) 1 and MRP3 attenuate cytotoxic and transactivating effects of the cyclopentenone prostaglandin, 15-deoxy-Δ12,14-prostaglandin J2 in MCF7 breast cancer cells. Biochemistry. 2003;42(18):5429–5437. doi: 10.1021/bi027347u. [DOI] [PubMed] [Google Scholar]
- 68.Murphy RC, Zarini S. Glutathione adducts of oxyeicosanoids. Prostaglandins & Other Lipid Mediators. 2002;68-69:471–482. doi: 10.1016/s0090-6980(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 69.O'Connor R. The pharmacology of cancer resistance. Anticancer Research. 2007;27(3A):1267–1272. [PubMed] [Google Scholar]
- 70.Olson RE. Discovery of the lipoproteins, their role in fat transport and their significance as risk factors. The Journal of Nutrition. 1998;128(2):439S–443S. doi: 10.1093/jn/128.2.439S. [DOI] [PubMed] [Google Scholar]
- 71.Vitols S, Peterson C, Larsson O, Holm P, Åberg B. Elevated uptake of low density lipoproteins by human lung cancer tissue in vivo. Cancer Research. 1992;52(22):6244–6247. [PubMed] [Google Scholar]
- 72.Lum DF, McQuaid KR, Gilbertson VL, Hughes-Fulford M. Coordinate up-regulation of low-density lipoprotein receptor and cyclo- oxygenase-2 gene expression in human colorectal cells and in colorectal adenocarcinoma biopsies. International Journal of Cancer. 1999;83(2):162–166. doi: 10.1002/(sici)1097-0215(19991008)83:2<162::aid-ijc3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. International Journal of Cancer. 2000;91(1):41–45. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 74.Edwards IJ, Berquin IM, Sun H, et al. Differential effects of delivery of omega-3 fatty acids to human cancer cells by low-density lipoproteins versus albumin. Clinical Cancer Research. 2004;10(24):8275–8283. doi: 10.1158/1078-0432.CCR-04-1357. [DOI] [PubMed] [Google Scholar]
- 75.Sun H, Berquin IM, Edwards IJ. Omega-3 polyunsaturated fatty acids regulate syndecan-1 expression in human breast cancer cells. Cancer Research. 2005;65(10):4442–4447. doi: 10.1158/0008-5472.CAN-04-4200. [DOI] [PubMed] [Google Scholar]
- 76.Sun H, Berquin IM, Owens RT, O'Flaherty JT, Edwards IJ. Peroxisome proliferator-activated receptor γ-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Research. 2008;68(8):2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heikkinen S, Auwerx J, Argmann CA. PPARγ in human and mouse physiology. Biochimica et Biophysica Acta. 2007;1771(8):999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-γ . Nature. 1998;396(6709):377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 79.Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-γ1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140(1):392–397. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 80.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochimica et Biophysica Acta. 2007;1771(8):952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Papageorgiou E, Pitulis N, Msaouel P, Lembessis P, Koutsilieris M. The non-genomic crosstalk between PPAR-γ ligands and ERK1/2 in cancer cell lines. Expert Opinion on Therapeutic Targets. 2007;11(8):1071–1085. doi: 10.1517/14728222.11.8.1071. [DOI] [PubMed] [Google Scholar]
- 82.Burgermeister E, Chuderland D, Hanoch T, Meyer M, Liscovitch M, Seger R. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor γ . Molecular and Cellular Biology. 2007;27(3):803–817. doi: 10.1128/MCB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pascual G, Fong AL, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ . Nature. 2005;437(7059):759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimizu M, Yamashita D, Yamaguchi T, Hirose F, Osumi T. Aspects of the regulatory mechanisms of PPAR functions: analysis of a bidirectional response element and regulation by sumoylation. Molecular and Cellular Biochemistry. 2006;286(1-2):33–42. doi: 10.1007/s11010-005-9052-z. [DOI] [PubMed] [Google Scholar]
- 85.Feige JN, Gelman L, Tudor C, Engelborghs Y, Wahli W, Desvergne B. Fluorescence imaging reveals the nuclear behavior of peroxisome proliferator-activated receptor/retinoid X receptor heterodimers in the absence and presence of ligand. The Journal of Biological Chemistry. 2005;280(18):17880–17890. doi: 10.1074/jbc.M500786200. [DOI] [PubMed] [Google Scholar]
- 86.Tudor C, Feige JN, Pingali H, et al. Association with coregulators is the major determinant governing peroxisome proliferator-activated receptor mobility in living cells. The Journal of Biological Chemistry. 2007;282(7):4417–4426. doi: 10.1074/jbc.M608172200. [DOI] [PubMed] [Google Scholar]
- 87.Powell E, Kuhn P, Xu W. Nuclear receptor cofactors in PPARγ-mediated adipogenesis and adipocyte energy metabolism. PPAR Research. 2007;2007:11 pages. doi: 10.1155/2007/53843. Article ID 53843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of nuclear translocation of HDAC3 by IκBα is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor γ function. The Journal of Biological Chemistry. 2006;281(7):4540–4547. doi: 10.1074/jbc.M507784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burgermeister E, Seger R. MAPK kinases as nucleo-cytoplasmic shuttles for PPARγ . Cell Cycle. 2007;6(13):1539–1548. doi: 10.4161/cc.6.13.4453. [DOI] [PubMed] [Google Scholar]
- 90.Kostadinova R, Wahli W, Michalik L. PPARs in diseases: control mechanisms of inflammation. Current Medicinal Chemistry. 2005;12(25):2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- 91.Zandbergen F, Mandard S, Escher P, et al. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochemical Journal. 2005;392(2):313–324. doi: 10.1042/BJ20050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Teunissen BEJ, Smeets PJH, Willemsen PHM, De Windt LJ, Van der Vusse GJ, Van Bilsen M. Activation of PPARδ inhibits cardiac fibroblast proliferation and the transdifferentiation into myofibroblasts. Cardiovascular Research. 2007;75(3):519–529. doi: 10.1016/j.cardiores.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 93.Bonofiglio D, Aquila S, Catalano S, et al. Peroxisome proliferator-activated receptor-γ activates p53 gene promoter binding to the nuclear factor-κB sequence in human MCF7 breast cancer cells. Molecular Endocrinology. 2006;20(12):3083–3092. doi: 10.1210/me.2006-0192. [DOI] [PubMed] [Google Scholar]
- 94.Ho T-C, Chen S-L, Yang Y-C, Liao C-L, Cheng H-C, Tsao Y-P. PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells. Cardiovascular Research. 2007;76(2):213–223. doi: 10.1016/j.cardiores.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 95.Zand H, Rhimipour A, Bakhshayesh M, Shafiee M, Nour Mohammadi I, Salimi S. Involvement of PPAR-γ and p53 in DHA-induced apoptosis in Reh cells. Molecular and Cellular Biochemistry. 2007;304(1-2):71–77. doi: 10.1007/s11010-007-9487-5. [DOI] [PubMed] [Google Scholar]
- 96.Edwards IJ, Sun H, Hu Y, et al. In vivo and in vitro regulation of syndecan 1 in prostate cells by n-3 polyunsaturated fatty acids. The Journal of Biological Chemistry. 2008;283(26):18441–18449. doi: 10.1074/jbc.M802107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor γ and β-catenin. Molecular and Cellular Biology. 2006;26(15):5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takada I, Suzawa M, Kato S. Nuclear receptors as targets for drug development: crosstalk between peroxisome proliferator-activated receptor γ and cytokines in bone marrow-derived mesenchymal stem cells. Journal of Pharmacological Sciences. 2005;97(2):184–189. doi: 10.1254/jphs.fmj04008x5. [DOI] [PubMed] [Google Scholar]
- 99.Rossi A, Kapahi P, Natoli G, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403(6765):103–118. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 100.Ackerman WE, IV, Zhang XL, Rovin BH, Kniss DA. Modulation of cytokine-induced cyclooxygenase 2 expression by PPARG ligands through NFκB signal disruption in human WISH and amnion cells. Biology of Reproduction. 2005;73(3):527–535. doi: 10.1095/biolreprod.104.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Medicinal Research Reviews. 2001;21(3):185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 102.Siavash H, Nikitakis NG, Sauk JJ. Abrogation of IL-6-mediated JAK signalling by the cyclopentenone prostaglandin 15d-PGJ2 in oral squamous carcinoma cells. British Journal of Cancer. 2004;91(6):1074–1080. doi: 10.1038/sj.bjc.6602055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siavash H, Nikitakis NG, Sauk JJ. Targeting of epidermal growth factor receptor by cyclopentenone prostaglandin 15-deoxy-Δ12,14-prostaglandin J2 in human oral squamous carcinoma cells. Cancer Letters. 2004;211(1):97–103. doi: 10.1016/j.canlet.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 104.Almishri W, Cossette C, Rokach J, Martin JG, Hamid Q, Powell WS. Effects of prostaglandin D2, 15-deoxy-Δ12,14-prostaglandin J2, and selective DP1 and DP2 receptor agonists on pulmonary infiltration of eosinophils in Brown Norway rats. Journal of Pharmacology and Experimental Therapeutics. 2005;313(1):64–69. doi: 10.1124/jpet.104.079079. [DOI] [PubMed] [Google Scholar]
- 105.O'Flaherty JT, Taylor JS, Thomas MJ. Receptors for the 5-oxo class of eicosanoids in neutrophils. The Journal of Biological Chemistry. 1998;273(49):32535–32541. doi: 10.1074/jbc.273.49.32535. [DOI] [PubMed] [Google Scholar]
- 106.Briscoe CP, Tadayyon M, Andrews JL, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. The Journal of Biological Chemistry. 2003;278(13):11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 107.Hirasawa A, Tsumaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature Medicine. 2005;11(1):90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 108.Katsuma S, Hatae N, Yano T, et al. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. The Journal of Biological Chemistry. 2005;280(20):19507–19515. doi: 10.1074/jbc.M412385200. [DOI] [PubMed] [Google Scholar]
- 109.Hughes-Fulford M, Li C-F, Boonyaratanakornkit J, Sayyah S. Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Research. 2006;66(3):1427–1433. doi: 10.1158/0008-5472.CAN-05-0914. [DOI] [PubMed] [Google Scholar]
- 110.Clay CE, Namen AM, Atsumi G-I, et al. Magnitude of peroxisome proliferator-activated receptor-γ activation is associated with important and seemingly opposite biological responses in breast cancer cells. Journal of Investigative Medicine. 2001;49(5):413–420. doi: 10.2310/6650.2001.33786. [DOI] [PubMed] [Google Scholar]
- 111.Gardner OS, Dewar BJ, Graves LM. Activation of mitogen-activated protein kinases by peroxisome proliferator-activated receptor ligands: an example of nongenomic signaling. Molecular Pharmacology. 2005;68(4):933–941. doi: 10.1124/mol.105.012260. [DOI] [PubMed] [Google Scholar]
- 112.Abedin M, Lim J, Tang TB, Park D, Demer LL, Tintut Y. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-γ pathways. Circulation Research. 2006;98(6):727–729. doi: 10.1161/01.RES.0000216009.68958.e6. [DOI] [PubMed] [Google Scholar]
- 113.O'Flaherty JT, Rogers LC, Chadwell BA, et al. 5(S)-hydroxy-6,8,11,14-E,Z,Z,Z-eicosatetraenoate stimulates PC3 cell signaling and growth by a receptor-dependent mechanism. Cancer Research. 2002;62(23):6817–6819. [PubMed] [Google Scholar]
- 114.Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and mediated by inhibition of translation initiation. Cancer Research. 2001;61(16):6213–6218. [PubMed] [Google Scholar]
- 115.Palakurthi SS, Flückiger R, Aktas H, et al. Inhibition of translation initiation mediates the anticancer effect of the n-3 polyunsaturated fatty acid eicosapentaenoic acid. Cancer Research. 2000;60(11):2919–2925. [PubMed] [Google Scholar]
- 116.Willermain F, Dulku S, Gonzalez NS, et al. 15-deoxy-12,14-prostaglandin J2 inhibits interferon gamma induced MHC class II but not class I expression on ARPE cells through a PPAR gamma independent mechanism. Prostaglandins & Other Lipid Mediators. 2006;80(3-4):136–143. doi: 10.1016/j.prostaglandins.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 117.Wilmer WA, Dixon C, Lu L, Hilbelink T, Rovin BH. A cyclopentenone prostaglandin activates mesangial MAP kinase independently of PPARγ . Biochemical and Biophysical Research Communications. 2001;281(1):57–62. doi: 10.1006/bbrc.2001.4301. [DOI] [PubMed] [Google Scholar]
- 118.Feinstein DL, Spagnolo A, Akar C, et al. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochemical Pharmacology. 2005;70(2):177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 119.Soller M, Dröse S, Brandt U, Brüne B, von Knethen A. Mechanism of thiazolidinedione-dependent cell death in Jurkat T cells. Molecular Pharmacology. 2007;71(6):1535–1544. doi: 10.1124/mol.107.034371. [DOI] [PubMed] [Google Scholar]
- 120.Chaffer CL, Thomas DM, Thompson EW, Williams ED. PPARγ-independent induction of growth arrest and apoptosis in prostate and bladder carcinoma. BMC Cancer. 2006;6, article 53:1–13. doi: 10.1186/1471-2407-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.MacLean CH, Newberry SJ, Mojica WA, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. Journal of the American Medical Association. 2006;295(4):403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 122.Kaizer L, Boyd NF, Kriukov V, Tritchler D. Fish consumption and breast cancer risk: an ecological study. Nutrition and Cancer. 1989;12(1):61–68. doi: 10.1080/01635588909514002. [DOI] [PubMed] [Google Scholar]
- 123.Hursting SD, Thornquist M, Henderson MM. Types of dietary fat and the incidence of cancer at five sites. Preventive Medicine. 1990;19(3):242–253. doi: 10.1016/0091-7435(90)90025-f. [DOI] [PubMed] [Google Scholar]
- 124.Sasaki S, Horacsek M, Kesteloot H. An ecological study of the relationship between dietary fat intake and breast cancer mortality. Preventive Medicine. 1993;22(2):187–202. doi: 10.1006/pmed.1993.1016. [DOI] [PubMed] [Google Scholar]
- 125.Terry PD, Terry JB, Rohan TE. Long-chain (n-3) fatty acid intake and risk of cancers of the breast and the prostate: recent epidemiological studies, biological mechanisms, and directions for future research. The Journal of Nutrition. 2004;134(12):3412S–3420S. doi: 10.1093/jn/134.12.3412S. [DOI] [PubMed] [Google Scholar]
- 126.Leitzmann MF, Stampfer MJ, Michaud DS, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. The American Journal of Clinical Nutrition. 2004;80(1):204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 127.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. British Journal of Cancer. 1996;74(1):159–164. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yeh C-C, Hsieh L-L, Tang R, Chang-Chieh CR, Sung F-C. Risk factors for colorectal cancer in Taiwan: a hospital-based case-control study. Journal of the Formosan Medical Association. 2003;102(5):305–312. [PubMed] [Google Scholar]
- 129.Yang C-X, Takezaki T, Hirose K, Inoue M, Huang X-E, Tajima K. Fish consumption and colorectal cancer: a case-reference study in Japan. European Journal of Cancer Prevention. 2003;12(2):109–115. doi: 10.1097/00008469-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 130.Simonsen N, van't Veer P, Strain JJ, et al. Adipose tissue omega-3 and omega-6 fatty acid content and breast cancer in the euramic study. American Journal of Epidemiology. 1998;147(4):342–352. doi: 10.1093/oxfordjournals.aje.a009456. [DOI] [PubMed] [Google Scholar]
- 131.Mamalakis G, Kafatos A, Kalogeropoulos N, Andrikopoulos N, Daskalopulos G, Kranidis A. Prostate cancer vs hyperplasia: relationships with prostatic and adipose tissue fatty acid composition. Prostaglandins Leukotrienes and Essential Fatty Acids. 2002;66(5-6):467–477. doi: 10.1054/plef.2002.0384. [DOI] [PubMed] [Google Scholar]
- 132.Freeman VL, Meydani M, Hur K, Flanigan RC. Inverse association between prostatic polyunsaturated fatty acid and risk of locally advanced prostate carcinoma. Cancer. 2004;101(12):2744–2754. doi: 10.1002/cncr.20676. [DOI] [PubMed] [Google Scholar]
- 133.Yang YJ, Lee SH, Hong SJ, Chung BC. Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia. Clinical Biochemistry. 1999;32(6):405–409. doi: 10.1016/s0009-9120(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 134.Norrish AE, Skeaff CM, Arribas GLB, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. British Journal of Cancer. 1999;81(7):1238–1242. doi: 10.1038/sj.bjc.6690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reddy BS, Cohen LA, McCoy GD, Hill P, Weisburger JH, Wynder EL. Nutrition and its relationship to cancer. Advances in Cancer Research. 1980;32:237–345. doi: 10.1016/s0065-230x(08)60363-2. [DOI] [PubMed] [Google Scholar]
- 136.Jurkowski JJ, Cave WT., Jr Dietary effects of menhaden oil on the growth and membrane lipid composition of rat mammary tumors. Journal of the National Cancer Institute. 1985;74(5):1145–1150. [PubMed] [Google Scholar]
- 137.Braden LM, Carroll KK. Dietary polyunsaturated fat in relation to mammary carcinogenesis in rats. Lipids. 1986;21(4):285–288. doi: 10.1007/BF02536414. [DOI] [PubMed] [Google Scholar]
- 138.Rose DP, Hatala MA, Connolly JM, Rayburn J. Effect of diets containing different levels of linoleic acid on human breast cancer growth and lung metastasis in nude mice. Cancer Research. 1993;53(18):4686–4690. [PubMed] [Google Scholar]
- 139.Rose DP, Connolly JM. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. Journal of the National Cancer Institute. 1993;85(21):1743–1747. doi: 10.1093/jnci/85.21.1743. [DOI] [PubMed] [Google Scholar]
- 140.Rose DP, Connolly JM, Rayburn J, Coleman M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. Journal of the National Cancer Institute. 1995;87(8):587–592. doi: 10.1093/jnci/87.8.587. [DOI] [PubMed] [Google Scholar]
- 141.Cannizzo F, Jr, Broitman SA. Postpromotional effects of dietary marine or safflower oils on large bowel or pulmonary implants of CT-26 in mice. Cancer Research. 1989;49(15):4289–4294. [PubMed] [Google Scholar]
- 142.Iigo M, Nakagawa T, Ishikawa C, et al. Inhibitory effects of docosahexaenoic acid on colon carcinoma 26 metastasis to the lung. British Journal of Cancer. 1997;75(5):650–655. doi: 10.1038/bjc.1997.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boudreau MD, Sohn KH, Rhee SH, Lee SW, Hunt JD, Hwang DH. Suppression of tumor cell growth both in nude mice and in culture by n-3 polyunsaturated fatty acids: mediation through cyclooxygenase-independent pathways. Cancer Research. 2001;61(4):1386–1391. [PubMed] [Google Scholar]
- 144.Reddy BS, Maruyama H. Effect of dietary fish oil on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Research. 1986;46(7):3367–3370. [PubMed] [Google Scholar]
- 145.Minoura T, Takata T, Sakaguchi M, et al. Effect of dietary eicosapentaenoic acid on azoxymethane-induced colon carcinogenesis in rats. Cancer Research. 1988;48(17):4790–4794. [PubMed] [Google Scholar]
- 146.Reddy BS, Sugie S. Effect of different levels of omega-3 and omega-6 fatty acids on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Research. 1988;48(23):6642–6647. [PubMed] [Google Scholar]
- 147.Takahashi M, Minamoto T, Yamashita N, Kato T, Yazawa K, Esumi H. Effect of docosahexaenoic acid on azoxymethane-induced colon carcinogenesis in rats. Cancer Letters. 1994;83(1-2):177–184. doi: 10.1016/0304-3835(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 148.Hendrickse CW, Keighley MR, Neoptolemos JP. Dietary ω-3 fats reduce proliferation and tumor yields at colorectal anastomosis in rats. Gastroenterology. 1995;109(2):431–439. doi: 10.1016/0016-5085(95)90330-5. [DOI] [PubMed] [Google Scholar]
- 149.Onogi N, Okuno M, Komaki C, et al. Suppressing effect of perilla oil on azoxymethane-induced foci of colonic aberrant crypts in rats. Carcinogenesis. 1996;17(6):1291–1296. doi: 10.1093/carcin/17.6.1291. [DOI] [PubMed] [Google Scholar]
- 150.Takahashi M, Fukutake M, Isoi T, et al. Suppression of azoxymethane-induced rat colon carcinoma development by a fish oil component, docosahexaenoic acid (DHA) Carcinogenesis. 1997;18(7):1337–1342. doi: 10.1093/carcin/18.7.1337. [DOI] [PubMed] [Google Scholar]
- 151.Paulsen JE, Stamm T, Alexander J. A fish oil-derived concentrate enriched in eicosapentaenoic and docosahexaenoic acid as ethyl esters inhibits the formation and growth of aberrant crypt foci in rat colon. Pharmacology & Toxicology. 1998;82(1):28–33. doi: 10.1111/j.1600-0773.1998.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 152.Karmali RA, Reichel P, Cohen LA, et al. The effects of dietary ω-3 fatty acids on the DU-145 transplantable human prostatic tumor. Anticancer Research. 1987;7(6):1173–1179. [PubMed] [Google Scholar]
- 153.Rose DP, Cohen LA. Effects of dietary menhaden oil and retinyl acetate on the growth of DU 145 human prostatic adenocarcinoma cells transplanted into athymic nude mice. Carcinogenesis. 1988;9(4):603–605. doi: 10.1093/carcin/9.4.603. [DOI] [PubMed] [Google Scholar]
- 154.Kobayashi N, Barnard RJ, Henning SM, et al. Effect of altering dietary ω-6/ω-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2 . Clinical Cancer Research. 2006;12(15):4662–4670. doi: 10.1158/1078-0432.CCR-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Berquin IM, Min Y, Wu R, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. The Journal of Clinical Investigation. 2007;117(7):1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacology & Therapeutics. 1999;83(3):217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 157.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor α and interleukin 1β production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. The American Journal of Clinical Nutrition. 1996;63(1):116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 158.Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR. Dietary ω-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. The Journal of Clinical Investigation. 1993;91(2):651–660. doi: 10.1172/JCI116245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Needleman P, Raz A, Minkes MS. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(2):944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mueller E, Sarraf P, Tontonoz P, et al. Terminal differentiation of human breast cancer through PPARγ . Molecular Cell. 1998;1(3):465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- 161.Mueller E, Smith M, Sarraf P, et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Elstner E, Müller C, Koshizuka K, et al. Ligands for peroxisome proliferator-activated receptory and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(15):8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Elstner E, Williamson EA, Zang C, et al. Novel therapeutic approach: ligands for PPARγ and retinoid receptors induce apoptosis in bcl-2-positive human breast cancer cells. Breast Cancer Research and Treatment. 2002;74(2):155–165. doi: 10.1023/a:1016114026769. [DOI] [PubMed] [Google Scholar]
- 164.Takashima T, Fujiwara Y, Higuchi K, et al. PPAR-gamma ligands inhibit growth of human esophageal adenocarcinoma cells through induction of apoptosis, cell cycle arrest and reduction of ornithine decarboxylase activity. International Journal of Oncology. 2001;19(3):465–471. [PubMed] [Google Scholar]
- 165.Allred CD, Talbert DR, Southard RC, Wang X, Kilgore MW. PPARγ1 as a molecular target of eicosapentaenoic acid in human colon cancer (HT-29) cells. The Journal of Nutrition. 2008;138(2):250–256. doi: 10.1093/jn/138.2.250. [DOI] [PubMed] [Google Scholar]
- 166.Lee JY, Hwang DH. Docosahexaenoic acid suppresses the activity of peroxisome proliferator-activated receptors in a colon tumor cell line. Biochemical and Biophysical Research Communications. 2002;298(5):667–674. doi: 10.1016/s0006-291x(02)02530-5. [DOI] [PubMed] [Google Scholar]
- 167.Trombetta A, Maggiora M, Martinasso G, Cotogni P, Canuto RA, Muzio G. Arachidonic and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chemico-Biological Interactions. 2007;165(3):239–250. doi: 10.1016/j.cbi.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 168.Allred CD, Kilgore MW. Selective activation of PPARγ in breast, colon, and lung cancer cell lines. Molecular and Cellular Endocrinology. 2005;235(1-2):21–29. doi: 10.1016/j.mce.2005.02.003. [DOI] [PubMed] [Google Scholar]