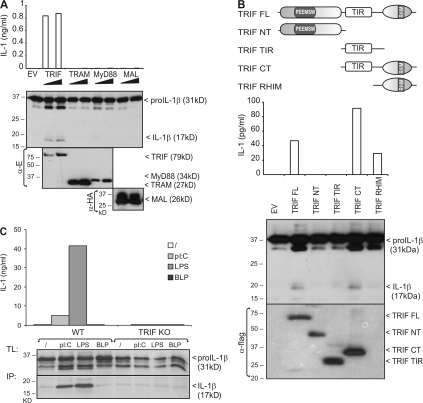
Figure 1.
Poly(I:C) and LPS induce pro–IL-1β processing via a TRIF-dependent signaling pathway. (A) HEK293T cells were cotransfected with pro–IL-1β and 50 or 100 ng of either E-TRIF, E-TRAM, E-MyD88, or HA-MAL. 24 h later, pro–IL-1β processing and expression of transfected proteins was analyzed by Western blotting of total cell lysates (bottom). Secretion of biologically active IL-1β into the corresponding cell supernatants was analyzed via IL-1 bioassay (top). (B) HEK293T cells were cotransfected with pro–IL-1β and different Flag-tagged TRIF deletion mutants. 24 h later, pro–IL-1β processing and secretion of biologically active IL-1β was analyzed as in A. Expression of TRIF mutants was verified by Western blotting and detection with anti-Flag. (top) Schematic representation of the different TRIF deletion mutants. FL, full length; NT, N-terminal fragment; CT, C-terminal fragment; PEEMSW, TRAF6-binding motif; TIR, Toll/IL-1 receptor domain. (C) BLP-primed peritoneal macrophages from WT and TRIF KO mice were incubated for 18 h with poly(I:C), LPS, BLP, or control medium, as described in the Materials and methods. Pro–IL-1β was analyzed in total cell lysates (TL) by Western blotting (bottom). Mature IL-1β was detected in the cell supernatant upon IL-1β immunoprecipitation (IP). Secretion of biologically active IL-1β was analyzed via IL-1 bioassay (top). Data are representative of three independent experiments.