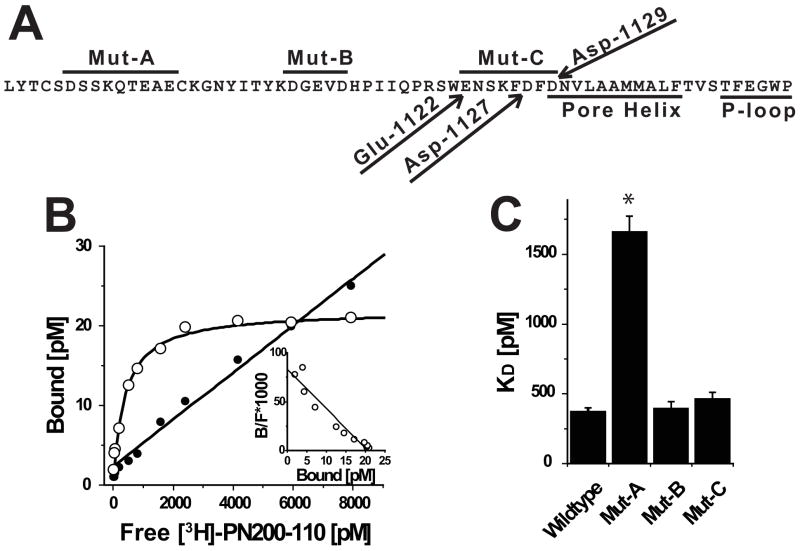
FIGURE 2. Acidic residues proximal to the extracellular portion of IIIS5 are important binding determinants for [3H]PN200-110.
(A) Amino acid sequence of CaV1.2 beginning at the C-terminus of IIIS5 through the pore-loop. Peptide segments corresponding to regions spanned by Mut-A, Mut-B and Mut-C are indicated by lines above sequence. Glu-1122, Asp-1127 and Asp-1129 are indicated with arrows. The Pore Helix and P-Loop are indicated by lines below the sequence. (B) Saturation binding experiment using membranes derived form cells expressing wild-type CaV1.2 channels was performed as described in the Materials and Methods. The signal-to-noise can be assessed by comparing the relative levels of specific (open circles) and non-specific (solid circles) binding. (inset) Scatchard transformation of data from Panel B indicates that the cells express a single population of high affinity receptor sites. (C) Similar analyses were performed on independent membrane preparations derived from cells expressing Wild-type (n = 6), Mut-A (n = 3), Mut-B (n = 3) and Mut-C (n = 3) channels (see Table 1).