Abstract
In the present study, we have analysed the phenotype of EphB2 and/or EphB3 deficient thymocytes confirming and extending previous studies on the role of this family of molecules in T-cell differentiation. In all mutant thymuses statistically significant reduced cell contents were observed. This reduction of thymic cellularity correlated with increased proportions of apoptotic cells, largely both double negative (DN; CD4− CD8−) and double positive (CD4+ CD8+) cells, and decreased proportions of DN cycling cells. Adult deficient thymuses also showed increased proportions of DN cells but not significant variations in the percentages of other thymocyte subsets. In absolute terms, the thymocyte number decreased significantly in all thymocyte compartments from the DN3 (CD44− CD25+) cell stage onward, without variations in the numbers of both DN1 (CD44+ CD25−) and DN2 (CD44+ CD25+) cells. Remarkably, all these changes also occurred from the 15-day fetal EphB2 and/or EphB3 deficient mice, suggesting that adult phenotype results from the gradual accumulations of defects appearing early in the thymus ontogeny. As a reflection of thymus condition, a reduction in the number of T lymphocytes occurred in the peripheral blood and mesenteric lymph nodes, but not in spleen, maintaining the proportions of T-cell subsets defined by CD4/CD8 marker expression, in all cases.
Keywords: Eph, ephrin, thymocyte differentiation, thymus
Introduction
Eph kinases are the largest family of cell surface receptor tyrosine kinases. In mammals, they comprise two groups of 10 EphA and six EphB receptors that bind other surface molecules called ephrinA (six members of glycosyl phosphatidylinositol-anchored proteins) and ephrinB (three members of transmembrane proteins). Both kinases and ligands transmit cytoplasmic signals and each receptor can bind several ligands and vice versa making this family of proteins a plastic and very complex biological system involved in a broad spectrum of cellular functions. In general, these molecules determine when and where the different cell types must move, attach or detach being, accordingly, involved in morphogenesis, cell positioning and cell migration. These functions are accomplished by regulating cytoskeleton dynamics, cell adhesion and integrin activity, but also by modulating gene expression and distinct intracellular pathways.1
Most Eph/ephrin have been detected in the thymus, a highly compartmentalized organ involved in T-cell maturation.2–4 Their roles and mechanisms of action in the organ are, however, controversial and largely unknown. We demonstrated that the interference of Eph/ephrinA signalling partially blocked T-cell differentiation and induced thymocyte death in rat fetal thymic organ cultures (FTOC).2 In a similar model, we recently showed that the addition of either EphB2-Fc or ephrinB1-Fc fusion proteins to FTOC decreased the number of both double positive (DP; CD4+ CD8+) and single positive (SP; both CD4+ CD8− and CD4− CD8+) thymocytes, in correlation with increased apoptosis.5In vivo, EphA4-deficient mice showed hypocellular thymuses with a decreased proportion of DP cells, accumulation of DN3 (CD4− CD8−; CD44+ CD25−) thymocytes and profound collapse of the cortical epithelial meshwork.6 On the contrary, other studies have reported that EphB6−/− mice exhibited normal cellularity with no structural alterations in the thymus although they did present compromised T-cell functions.7,8 But EphB6 overexpression in transgenic mice resulted in breakdown of the thymic cortex–medulla limits and alterations in the splenic white pulp.9 On the other hand, ephrinB1 has been reported to be critical for T-cell development. EphrinB1 knock-down mice appear to have significantly altered T-cell development10 and murine FTOC that grew in the presence of ephrinB1–Fc fusion proteins contained a decreased proportion of DP cells and increased percentage of both SP thymocytes.11 However, a thymus phenotype has not been found in mice deficient in EphB2, one of the main receptors of ephrinB1.9
In order to extend and clarify the presumptive role of Eph/ephrin in thymus development, we have examined the in vivo phenotype of deficient mice for either EphB2 and/or EphB3. The first studies associated these Eph kinases and their ligands with the guidance of axons and palate formation.12–14 Further results demonstrated their involvement in the control of cell positioning in the intestinal epithelium,15 vascular and urorectal development,16,17 in the co-ordination of migration and proliferation in the intestinal stem cell niche18 and tumorigenesis, especially of epithelial origin.19–23
Both EphB2 and EphB324 as well as their main ligands, ephrinB1 and ephrinB2, have been reported to be expressed in total thymocytes25–29 and could be involved in T-cell activation. Both EphB co-migrate with T-cell receptor (TCR) to lipid rafts after treatment with anti-CD3 monoclonal antibody (mAb)4 and cross-linking of EphB2 receptors with ephrinB1 in mouse thymocytes protects them from anti-CD3 antibody-induced apoptosis.30 After TCR ligation, solid-phase ephrinB1 stimulates T-cell responses, including cell proliferation, lymphokine production and cytotoxic T lymphocyte activity.10 However, the stimulation of CD3+ thymocytes with ephrinB1 blocks the main responses induced via TCR, including interleukin-2 production and up-regulated CD25 expression.30 Recently, we extended these results demonstrating that immobilized EphB2-Fc and ephrinB1-Fc modulate anti-CD3 antibody induced apoptosis of DP thymocytes in a process dependent on concentration.5 Moreover, ephrinB1 and ephrinB2 inhibit Jurkat and normal T-cell chemotaxis induced by stromal-derived factor-1α.24,31,32
In the current work we show that the lack of EphB2 and/or EphB3 in mice results in an important decrease in thymic cellularity and slight variations in the relative proportions of thymocyte subpopulations, largely affecting the double negative (DN; CD4− CD8−) compartment confirming previous data that suggested a role for Eph and ephrin in thymus biology.
Materials and methods
Mice
EphB2- and/or EphB3-deficient mice in a CD1 background were provided by Dr Mark Henkemeyer (University of Texas, Southwestern Medical Center at Dallas, Dallas, TX). The day of vaginal plug detection was designated as day 0·5. All animals were bred and maintained under pathogen-free conditions in the Complutense University of Madrid facilities. Descendents from heterozygous parents were used for analysis in all cases.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis
Thymocyte cell suspensions were labelled with mAbs CD4-Tricolor, CD8αβ-antigen-presenting cell (APC; Caltag Laboratories, Invitrogen, Barcelona, Spain) and TCRαβ–fluoroscein isothiocyanate (FITC; BD Biosciences, Erembodegem, Belgium). Thymocyte subsets [CD4− CD8− TCRαβ− (DN), CD4+ CD8+ TCRαβ−/+ (DP), CD4+ CD8− TCRαβhi (CD4) and CD4− CD8+ TCRαβhi (CD8)] were obtained in a sorter FACSVANTAGE SE (BD Immunocytometry systems). Enrichment in thymic epithelial cell suspensions was developed as previously described.33 To remove endogenous thymocytes, total thymic epithelial cell suspensions were labelled with mAb anti-CD45 (BD Biosciences) and then labelled with a biotinylated anti-rat immunoglobulin G (IgG; Jackson Immunoresearch Laboratories, West Grove, PA). The cells were then washed, labelled with avidin Dynabeads (Dynabeads, Dynal, Norway) according to the manufacturer's specifications and negatively selected. Negative cell fractions were constituted by thymic epithelial cells that, in all cases, contained >99% purity. Isolation of RNA from thymus organ, thymocyte subsets and total thymic epithelial cells was carried out with tri-reagent (Sigma-Aldrich, St Louis, MO) according to the manufacturer's specifications. cDNA synthesis was developed with Superscript III RT kit (Invitrogen) according to manufacturer's specifications. PCR primer sequences were obtained using Primer3 software from EphB2, EphB3, ephrinB1, ephrinB2 and β-actin (control for cDNA quality) cDNA sequences from GenBank databases. The following primers were used: EphB2, forward 5′-CTGCCACCAGCGAAGTGC-3′, reverse 5′-GAGCTGGGCTGGATGGTA-3′ (571bp); EphB3, forward 5′-GCTTCTGCCGCTGCTCGCTCC-3′, reverse 5′-GAAGCCTGCAGTGGTGGATGC-3′ (607 bp); ephrinB1, forward 5′-TGCTAGGGGATCCTGAAGTG-3′, reverse 5′-TGCGGAGCTTGAGTAGTAGGA-3′ (833bp) and ephrinB2, forward 5′-AGAACTGGGAGCGGCTTG-3′, reverse 5′-GGTGTCTCCTGCGGTACTTG-3′ (799 bp). As a control for cDNA quality we performed β-actin amplification, forward: 5′-AGAGATGGCCACGGCTGCTT-3′, reverse: 5′-TTTGCGGTGGACGATGGAG-3′ (445 bp). The PCR conditions were: EphB2 and β-actin, 94° for 3 min, 94° for 45 s, 60° for 45 s, 72° for 45 s for 40 cycles and then 72° for 10 min; EphB3, 94° for 3 min, 94° for 45 s, 55° for 45 s, 72° for 45 s for 40 cycles and then 72° for 10 min; ephrinB1 and ephrinB2, 94° for 3 min, 94° for 45 s, 57° for 45 s, 72° for 45 s for 40 cycles and then 72° for 10 min. The amplification products were analyzed by 1·5% agarose electrophoresis.
Immunofluorescence
Cryosections 6 μm thick from either wild type (WT) or mutant mice were fixed in acetone for 10 min and air dried. Slides were stained with either anti-EphB2, anti-EphB3, anti-ephrinB2 (R&D Systems, Oxford, UK) and anti-ephrinB1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-keratin5 (K5) (Covance), anti-keratin8 (K8, Troma-1; Developmental Studies Hybridoma Bank). Primary antibodies were incubated for 1 hr at room temperature and detected using anti-rabbit IgG-Alexa-fluor 488, anti-rat IgG-aminomethylcoumarin (AMCA), and anti-goat IgG–FITC (Molecular Probes, Invitrogen, Barcelona, Spain). Secondary antibodies were incubated for 45 min at room temperature and then sections were washed in cold phosphate-buffered saline (PBS) three times for 5 min. Finally, sections were mounted in Antifade Prolong Gold (Molecular Probes, Invitrogen). Sections were analysed using a Zeiss Axioplan microscope, photographed with a Spot 2 digital camera and analysed using Metamorph software (MDS Inc., Toronto, Canada) at the Microscopy and Cytometry Centre (Complutense University, Madrid, Spain).
Flow cytometry
Thymus cell suspensions were stained for 15 min in PBS 1% fetal calf serum (FCS) with specific mAbs against either CD4-Tricolor, CD8αβ-APC (Caltag Laboratories, Invitrogen), TCRαβ–FITC, TCRγδ–PE, CD25–PerCpCy5.5, CD44–FITC, CD117 (c-Kit-PE) and lineage cocktail (Lin: CD3ε, CD11b, CD45R/B220, Ly-76, Ly-6G and Ly-6C)–APC (BD Biosciences). DN subset analysis, from a Lin subpopulation, was carried out as previously described.34 Peripheral lymphocyte suspensions from spleen, mesenteric lymph nodes and peripheral blood were stained for 15 min in PBS 1% FCS with specific mAbs against either CD4–PE, CD8α–APC (Caltag Laboratories, Invitrogen). After staining, cell suspensions were washed and resuspended for analysis. Flow cytometric analysis was performed using a FACSCalibur (BD Biosciences) and CellQuest software at the Microscopy and Cytometry Centre (Complutense University, Madrid, Spain). Non-viable cells were excluded by forward and side scatter (FSC and SSC). In each experimental condition data represent the mean ± SD of at least five independent animals. The significance of the Student's t-test probability is indicated as: *P < 0·05; **P < 0·01 and ***P < 0·005.
Cell cycle and cell death analysis
For cell cycle analysis, after staining with the above-mentioned specific mAbs, cells were fixed overnight in Cellfix (BD Biosciences) and stained with Hoechst 33342 (Molecular Probes) in ethanol 30% in PBS 1% bovine serum albumin for 30 min at room temperature. For cell death analysis, thymocyte suspensions were stained with the above indicated specific mAb and Annexin-V–FITC (Roche Diagnostics, Basel, Switzerland) in HEPES buffer 1% FCS for 20 min at 4°. Cell suspensions were stained with propidium iodide (PI) to discard complete cell death from apoptotic cells. Apoptotic cells were defined as Annexin-V+/PI−. At least 20 000 cells/sample were analyzed for cell cycle and cell death analysis in LSR and FACSCalibur (BD Immunocytometry Systems), respectively, and CellQuest software at the Microscopy and Cytometry Centre (Complutense University, Madrid, Spain). Non-viable cells were excluded by FSC and SSC in all cases.
Results
EphB2- and EphB3-deficient mice were viable, fertile and normal, although some EphB2 mutants sometimes showed a cycling behaviour. On the contrary, EphB2/B3 double mutants had low survival, confirming previous reports14 and some surviving ones were infertile because of problems in the development of the urogenital system.16
EphB2, EphB3, ephrinB1 and ephrinB2 are expressed in the thymus
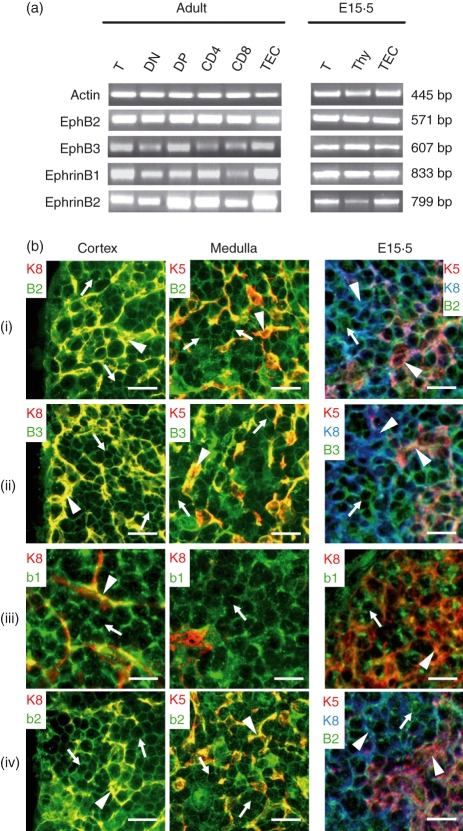
Both EphB2 and EphB3 and their main ligands, ephrinB1 and ephrinB2, were expressed in total thymocytes as well as in the different CD4/CD8 defined thymocyte subsets from adult and fetal mice, as shown by RT–PCR analysis (Fig. 1a). Also, the thymic epithelium expressed the two EphB receptors and their ligands (Fig. 1a). For further topological location of the four molecules, we carried out an immunofluorescence study on thymus cryosections that confirmed their expression on both thymocytes and thymic epithelial cells (TEC) of thymic cortex and medulla (Fig. 1b). The combination of specific reagents for detecting either Eph/ephrinB or cortical (keratin 8+ cells, K8) and medullary epithelial cells (keratin 5+ cells; K5) allowed the expression of EphB2 and EphB3 to be determined in both epithelial cells (Fig. 1b arrowhead) and thymocytes (Fig. 1b arrow, keratin negative cells). Because specific antibodies for both ephrinB1 and K5 have the same origin and isotype, ephrinB1 expression in the medullary epithelium was indirectly demonstrated as ephrinB1 positive cells occurring in K8 negative medullary epithelial areas (Fig. 1b iii). Moreover, in 15·5-day fetal thymus (E15·5), the four molecules were already expressed in both thymocytes and epithelial cells (Fig. 1b, E15·5).
Figure 1.
Expression of EphB receptors and ephrinB ligands in mouse thymus. (a) Specific primer pairs were used to determine by RT–PCR analysis the presence of EphB2, EphB3, ephrinB1 and ephrinB2 in total thymus (T), total thymocytes (Thy) isolated thymocyte subpopulations based on CD4, CD8, and TCRαβ expression. (DN: CD4− CD8− TCRαβ−; DP: CD4+ CD8+ TCRαβ+/−; CD4: CD4+ CD8− TCRαβ+; CD8: CD4− CD8+ TCRαβ+) and isolated thymic epithelial cells (TEC). Samples were obtained from adult or 15.5 embryonic (E15.5) WT mice. β-actin served as positive control. Corresponding band sizes are indicated on the right side of the figure. (b) Topological detection of Eph/ephrinB on thymus cryosections from adult and 15.5 embryonic (E15.5) WT mice. EphB2 (i), EphB3 (ii), ephrinB1 (b1) (iii) and ephrinB2 (b2) (iv) are expressed on both thymocytes (keratin negative cells; arrows) and epithelial cells (arrowheads) of thymic cortex (K8+ cells) and medulla (K5+ cells). Scale bar 10 μm.
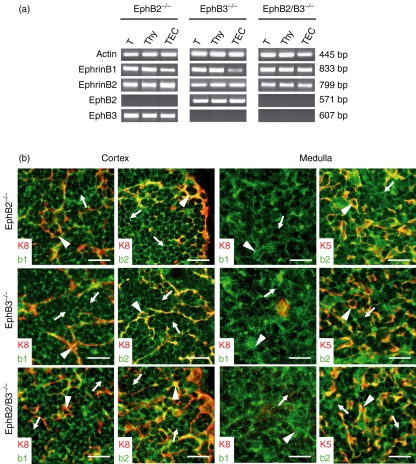
On the other hand, as shown by RT–PCR analysis, EphB-deficient mice did not express the respective knocked molecule but they did express the other Eph and ephrin studied (Fig. 2a). In this respect, despite the profound alterations found in the thymus cytoarchitecture of EphB-deficient mice (J. García-Ceca, E. Jiménez, D. Alfaro, T. Cejalvo, J. J. Muñoz, A. Zapata, unpublished data), ephrinB expression remained unaltered on both thymocytes and thymic epithelial cells (Fig. 2b). The thymic phenotype observed in the EphB-deficient mice must, therefore, be accounted for by the absence of respective receptors, EphB2 and/or EphB3, rather than to the altered expression of other Eph and/or ephrin.
Figure 2.
Expression of Eph/ephrinB in the thymus of EphB2 and/or EphB3-deficient mice. (a) The presence of EphB2, EphB3, ephrinB1 and ephrinB2 was determined by RT–PCR in thymus (T), total thymocytes (Thy) and thymic epithelial cells (TEC) from EphB2 and/or EphB3-deficient mice. All EphB-deficient mice kept the expression of the other Eph/ephrinsB analyzed. Corresponding band sizes are indicated on the right side of the figure. (b) Topological detection of ephrinB1 (b1) and ephrinB2 (b2) in the thymic cortex and medulla defined by the expression of K8 and K5 keratins respectively from EphB2 (EphB2−/−), EphB3 (EphB3−/−) and EphB2/B3 (EphB2/B3−/−) deficient mice. Arrowheads label the ephrinB expression on thymic epithelial cells from the cortex (K8+ cells) or medulla (K5+ cells). EphrinB positive cells are marked by arrows. Scale bar 20 μm.
EphB-deficient mice present decreased cell numbers and slightly altered proportions of thymocyte subsets
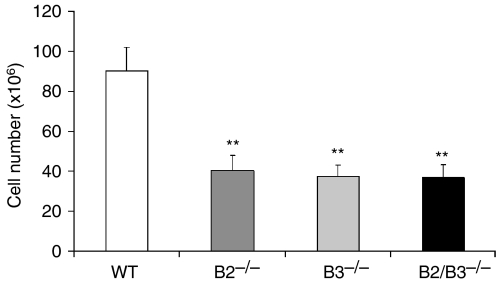
In all deficient mice studied, the thymic cellularity significantly decreased compared to the numbers of thymic cells found in the control, WT mice (Fig. 3). Nevertheless, the EphB-deficient mice only showed slight variations in the proportions of different thymocyte subsets, defined by the expression of CD4/CD8 cell markers, in comparison with the values found in the WT mice, largely consisting of increased proportions of both DN cells and TCRγδ thymocytes (Table 1). In absolute terms, there was, however, a general decrease in the numbers of all thymocyte compartments, the most important occurring in both DP and SP thymocytes, whereas the absolute numbers of DN cells and TCRγδ thymocytes remained unchanged (Table 2). These results demonstrate a low efficiency in the TCRαβ thymocyte production from the first stages of T-cell maturation in EphB-deficient mice that results in a slight accumulation of the percentage of DN thymocytes and decreased numbers of both DP and SP thymocytes without changes in the number of TCRγδ cells.
Figure 3.
Analysis of the thymocyte number in EphB2 and/or EphB3 mutant mice. Thymic cellularity showed a statistically significant decrease in EphB2 (B2−/−), EphB3 (B3−/−) and EphB2/B3 (B2/B3−/−) deficient mice as compared with WT mice.
Table 1.
Percentages of CD4/CD8 thymocyte subsets and TCRγδ cells in adult WT and EphB-deficient mice
| DN | DP | CD4 | CD8 | TCRγδ | |
|---|---|---|---|---|---|
| Wild type | 5·13 ± 1·39 | 75·73 ± 2·62 | 15·88 ± 0·82 | 3·26 ± 0·60 | 0·38 ± 0·02 |
| EphB2−/− | 8·54 ± 1·40* | 73·67 ± 3·41 | 14·76 ± 2·16 | 3·03 ± 0·91 | 0·80 ± 0·17* |
| EphB3−/− | 8·80 ± 2·57* | 73·14 ± 4·77 | 14·62 ± 2·73 | 3·44 ± 2·51 | 0·81 ± 0·12* |
| EphB2/B3−/− | 8·41 ± 1·96* | 72·96 ± 3·08 | 15·10 ± 1·49 | 3·53 ± 0·15 | 0·91 ± 0·18* |
Showed data are means ± standard deviations.
P < 0·05.
Table 2.
Absolute numbers (×106) of CD4/CD8 thymocyte subsets and TCRγδ cells in adult WT and EphB-deficient mice
| DN | DP | CD4 | CD8 | TCRγδ | |
|---|---|---|---|---|---|
| Wild type | 4·64 ± 1·26 | 68·46 ± 2·37 | 14·36 ± 0·75 | 2·95 ± 0·54 | 0·35 ± 0·01 |
| EphB2−/− | 3·44 ± 0·56 | 29·69 ± 1·37*** | 5·95 ± 0·87*** | 1·22 ± 0·37** | 0·32 ± 0·07 |
| EphB3−/− | 3·31 ± 0·97 | 27·50 ± 1·79*** | 5·50 ± 1·03*** | 1·29 ± 0·94** | 0·30 ± 0·05 |
| EphB2/B3−/− | 3·09 ± 0·72 | 26·85 ± 1·13*** | 5·56 ± 0·55*** | 1·30 ± 0·06** | 0·33 ± 0·07 |
Showed data are means ± standard deviations.
P < 0·01 and
P < 0·005.
In order to further analyse the variations found in the EphB-deficient DN thymocytes, a heterogeneous cell population that contains cells belonging to different lymphoid and non-lymphoid cell lineages, we evaluated the expression of c-Kit, CD44 and CD25 in the thymic lineage negative (Lin−) cell subpopulation, as described in the Material and methods section. The results demonstrated a statistically significant decrease in the proportion of DN3 (c-Kitlo CD44− CD25+) cells in all deficient mice studied (Table 3). On the contrary, the proportions of DN1 (c-Kit+ CD44+ CD25−) cells increased with significant differences to control values in EphB3- and EphB2/B3-deficient mice but not in the EphB2 mutants. Moreover, the percentage of DN4 (c-Kit− CD44− CD25−) cells significantly increased in the thymus of double mutant mice (Table 3).
Table 3.
Proportions of DN cell subsets defined by CD44/CD25 expression in adult WT and EphB-deficient mice
| DN1 | DN2 | DN3 | DN4 | |
|---|---|---|---|---|
| Wild type | 2·67 ± 0·45 | 1·49 ± 0·66 | 63·46 ± 3·36 | 32·11 ± 3·64 |
| EphB2−/− | 3·38 ± 1·55 | 1·57 ± 0·51 | 55·85 ± 1·67* | 32·47 ± 7·20 |
| EphB3−/− | 6·19 ± 1·30*** | 1·22 ± 0·59 | 56·20 ± 3·43* | 33·28 ± 3·32 |
| EphB2/B3−/− | 6·83 ± 2·04** | 1·09 ± 0·41 | 50·93 ± 6·70* | 37·06 ± 5·95* |
Showed data are means ± standard deviations.
P < 0·05
P < 0·01 and
P < 0·005.
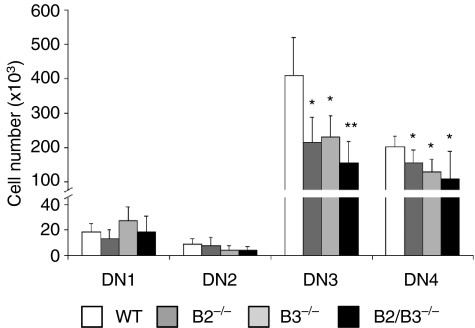
In absolute terms, although the thymic cellularity was extremely reduced in the EphB-deficient mice, the numbers of both DN1 and DN2 (c-Kit+ CD44+ CD25+) cells were similar to those of WT mice, but a significantly lower number of thymocytes reached the next T-cell stages, DN3, DN4 (Fig. 4), DP, etc. All these results, therefore, suggest that the lack of EphB2 and/or EphB3 in the mutant mice alters the efficiency of intrathymic T-cell differentiation from the DN2 cell compartment onward, resulting in a lower production of thymocytes in these than in WT mice.
Figure 4.
Variations in the numbers of DN (CD4− CD8−) subsets defined by CD44/CD25 expression. Both DN3 (CD44− CD25+) and DN4 (CD44− CD25−) cell numbers significantly decreased in EphB2 (B2−/−)-, EphB3 (B3−/−)- and EphB2/B3 (B2/B3−/−)-deficient mice.
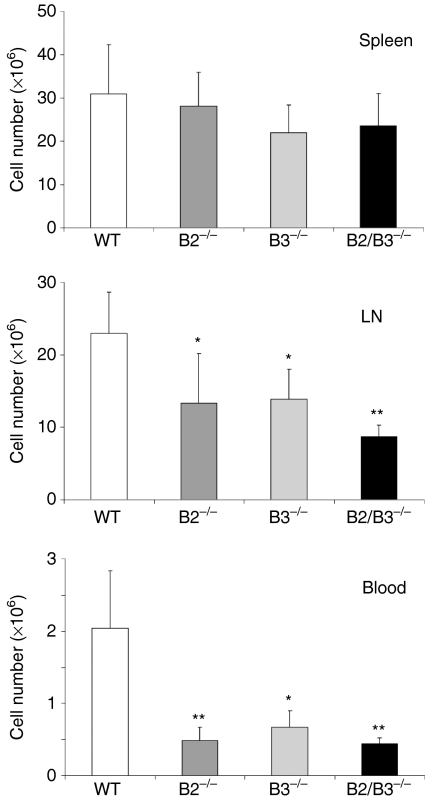
The number of peripheral T lymphocytes fluctuated in the deficient mice. In all mutants, both peripheral blood and mesenteric lymph nodes, but not spleen, the absolute T-cell number was significantly lower than in WT (Fig. 5). Nevertheless, these numerical variations did not result in statistically significant differences in the percentage of peripheral CD4/CD8 defined T-cell subpopulations from EphB-deficient mice, compared with the control values observed in WT mice (Table 4).
Figure 5.
Variations in the T-cell number of peripheral lymphoid organs of EphB-deficient mice. Statistically significant differences occurred in the numbers of peripheral T lymphocytes of both peripheral blood and mesenteric lymph nodes (LN), but not of the spleen of EphB2 (B2−/−)-, EphB3 (B3−/−)- and EphB2/B3 (B2/B3−/−)-deficient mice, as compared to control values observed in WT mice.
Table 4.
Proportions of peripheral CD4/CD8 T lymphocyte subsets in spleen, mesenteric lymph nodes and peripheral blood of adult WT and EphB-deficient mice
| DP | CD4 | CD8 | |
|---|---|---|---|
| (a) Spleen | |||
| Wild type | 0·43 ± 0·08 | 79·82 ± 2·20 | 18·88 ± 2·07 |
| EphB2−/− | 0·61 ± 0·11 | 78·15 ± 3·91 | 20·49 ± 3·02 |
| EphB3−/− | 0·59 ± 0·12 | 79·01 ± 3·85 | 19·47 ± 3·38 |
| EphB2/B3−/− | 0·52 ± 0·10 | 77·23 ± 3·06 | 21·40 ± 2·74 |
| (b) Lymph nodes | |||
| Wild type | 0·60 ± 0·07 | 79·44 ± 2·37 | 19·15 ± 2·39 |
| EphB2−/− | 0·93 ± 0·19 | 77·92 ± 3·64 | 20·74 ± 3·46 |
| EphB3−/− | 0·66 ± 0·17 | 79·11 ± 3·01 | 19·31 ± 2·64 |
| EphB2/B3−/− | 0·69 ± 0·21 | 79·26 ± 3·28 | 19·67 ± 2·85 |
| (c) Peripheral blood | |||
| Wild type | 0·21 ± 0·06 | 79·61 ± 4·18 | 19·44 ± 3·20 |
| EphB2−/− | 0·18 ± 0·04 | 80·11 ± 4·76 | 18·92 ± 3·19 |
| EphB3−/− | 0·23 ± 0·09 | 79·70 ± 4·05 | 19·33 ± 4·20 |
| EphB2/B3−/− | 0·17 ± 0·05 | 78·47 ± 3·89 | 20·71 ± 3·53 |
Showed data are means ± standard deviations.
Decreased thymocyte number observed in the EphB-deficient mice correlate with variations in the proportions of both apoptotic and cycling thymic cells
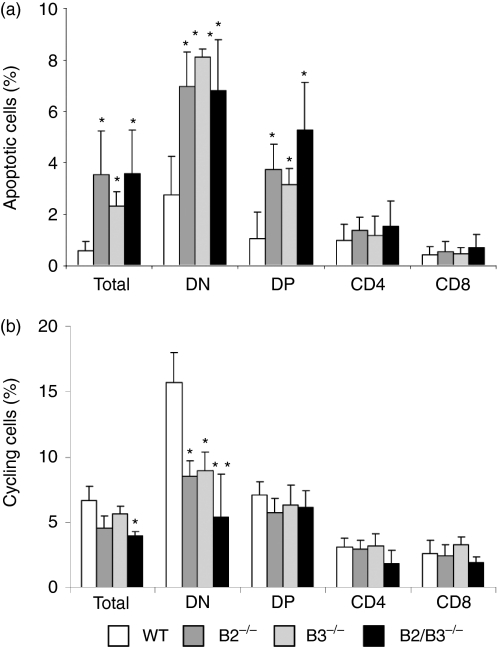
The three EphB-deficient mice studied showed statistically significant increases in the total number of apoptotic cells (Annexin V+) (Fig. 6a). All thymocyte subsets showed increased numbers of apoptotic cells but only the DN and DP cell compartments exhibited statistically significant variations compared to control values.
Figure 6.
Changes in the proportions of apoptotic (a) and cycling thymocytes (b) of mutant mice compared with control WT values. (a) Annexin-V staining in combination with CD4/CD8 cell marker expression was used to determine the percentage of apoptotic cells within the different thymic subpopulations. Dead cells were excluded by using propidium iodide (PI) staining. The percentage of apoptotic cells (mean ± SD) increased significantly in total thymocytes (total) as well as in both DN and DP cells, but not in the SP cell subpopulations of mutant mice. (b) Cell cycle in both WT and mutant mice was analyzed by combination of Hoechst 33342 and CD4/CD8 cell marker expression. The percentage (mean ± SD) of cells in S+G2+M phases is represented. Note the decreased proportion of cycling cells that occurs in the DN (CD4− CD8−) cell compartment but not in the other thymocyte subsets of mutant thymuses.
On the other hand, the percentage of total cycling thymic cells also showed reduced values compared to controls, although statistically significant differences only occurred in the double EphB2/B3 defective mice. However, a significant decrease was found in the proportion of cycling DN thymocytes in the three defective mice analysed, whereas the DP and SP cell compartments were not affected (Fig. 6b).
The alterations observed in the thymus of EphB-deficient mice appear early in development
In order to further evaluate the origin of observed phenotype in the adult EphB-deficient thymuses we looked for possible alterations in both cell content and T-cell maturation of neonatal and fetal mutant mice. Remarkably, thymic changes observed in adult deficient mice occurred already in the neonatal and fetal animals.
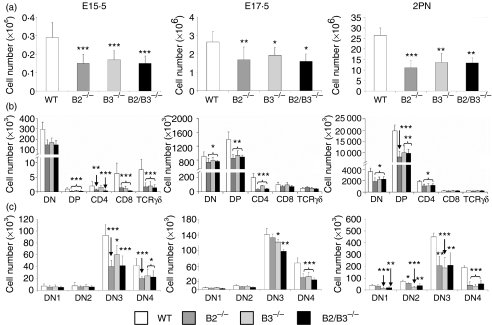
At all evaluated stages (15·5 and 17·5-day fetal and 2 days postnatal), the deficient thymuses showed significantly lower cellularity than the WT ones of the same ages (Fig. 7a). We also found that the proportions of DN cells were significantly higher in the EphB-deficient mice studied than in the control WT mice (Table 5). The other T-cell subpopulations defined by CD4/CD8 cell marker expression in general, underwent a statistically significant reduction, largely in fetal (Table 5a, b) but not in postnatal mice (Table 5c). The proportions of TCRγδ cells decreased significantly in the E15·5 mutant thymus, to significantly in both E17·5 and postnatal thymuses (Table 5). At 15·5 days of fetal life the DN1 and DN2 subsets underwent statistically significant increased percentages whereas the relative proportions of DN3 cells significantly diminished in the three studied mutants (Table 6a). At 17·5 days of fetal life the proportions of DN3 cells increased in EphB-deficient mice whereas those of DN4 thymocytes were reduced and no variations occurred in the percentages of both DN1 and DN2 cells (Table 6b). The situation was similar in postnatal mice although in these animals the proportions of DN1 cells increased significantly in the three deficient mice studied (Table 6c). In absolute terms, the numbers of all thymocyte subsets, including TCRγδ cells, significantly decreased in the E15·5 mutant thymuses whereas in both E17·5 and 2 days postnatal deficient thymuses the reduction only affected the DN, DP, and SP CD4+ CD8− cell compartments (Fig. 7b). Within the DN cell compartment, the number of DN3 and DN4 cells decreased in the three analysed stages as found above in the adult thymus (Fig. 7c). In the 2 days postnatal EphB-deficient thymuses, the numbers of DN1 and DN2 cells also decreased (Fig. 7c).
Figure 7.
Variations in the numbers of total thymic cells and thymocyte subsets occur in neonatal and fetal mutant mice. (a) Both fetal (E15.5 and E17.5) and neonatal mice showed reduced numbers of total thymic cells as compared to WT mice of the same age. (b) In all analysed stages (E15.5, E17.5, 2PN) the numbers of different thymocyte subsets defined by CD4/CD8 expression decreased in the mutant mice with significant variations largely affecting DN, DP and SP CD4+ CD8− cell compartments. (c) At any analysed stages, decreased numbers of DN3 and DN4 cells occurred in the EphB-deficient thymus. In the 2 days postnatal mutant thymuses (2PN) the absolute numbers of both DN1 and DN2 cells were also lower than those of control WT thymuses.
Table 5.
Percentages of CD4/CD8 thymocyte subsets and TCRγδ cells in 15·5-day fetal (E15·5), 17.5-day fetal (E17·5) and neonatal (2PN) WT and EphB-deficient mice
| DN | DP | CD4 | CD8 | TCRγδ | |
|---|---|---|---|---|---|
| (a) E15·5 | |||||
| Wild type | 95·08 ± 1·08 | 0·24 ± 0·18 | 0·61 ± 0·35 | 3·10 ± 1·09 | 2·09 ± 0·80 |
| EphB2−/− | 98·27 ± 1·80*** | 0·07 ± 0·04*** | 0·40 ± 0·13* | 0·91 ± 0·39*** | 1·09 ± 0·77*** |
| EphB3−/− | 98·63 ± 0·79*** | 0·03 ± 0·03*** | 0·61 ± 0·29 | 0·54 ± 0·41*** | 1·22 ± 0·83** |
| EphB2/B3−/− | 98·95 ± 1·06*** | 0·04 ± 0·02*** | 0·19 ± 0·13*** | 0·18 ± 0·03*** | 0·97 ± 0·52*** |
| (b) E17·5 | |||||
| Wild type | 32·89 ± 4·01 | 54·84 ± 3·26 | 7·49 ± 2·72 | 3·85 ± 1·21 | 1·91 ± 0·46 |
| EphB2−/− | 43·42 ± 2·91*** | 49·62 ± 3·36* | 1·81 ± 0·68*** | 4·33 ± 0·92 | 3·00 ± 1·16** |
| EphB3−/− | 41·85 ± 3·91** | 48·75 ± 1·19** | 4·12 ± 0·25*** | 4·92 ± 1·56 | 2·59 ± 0·54* |
| EphB2/B3−/− | 42·84 ± 2·35*** | 50·32 ± 2·44* | 2·26 ± 0·38*** | 4·26 ± 0·64 | 2·32 ± 0·15** |
| (c) 2PN | |||||
| Wild type | 13·65 ± 0·69 | 74·30 ± 0·73 | 9·79 ± 1·40 | 1·51 ± 0·24 | 1·02 ± 0·29 |
| EphB2−/− | 16·52 ± 1·37** | 71·14 ± 3·25 | 10·09 ± 2·61 | 1·56 ± 0·38 | 2·81 ± 0·82** |
| EphB3−/− | 16·55 ± 1·69* | 72·99 ± 2·54 | 8·13 ± 0·65 | 1·49 ± 0·25 | 2·49 ± 0·27*** |
| EphB2/B3−/− | 17·17 ± 1·07*** | 71·91 ± 2·87 | 8·90 ± 1·03 | 1·58 ± 0·30 | 2·51 ± 0·10** |
Showed data are means ± standard deviations.
P < 0.05
P < 0.01 and
P < 0.005.
Table 6.
Proportions of DN cell subsets, defined by CD44/CD25 expression, in 15·5-day fetal (E15·5), 17·5-day fetal (E17·5) and neonatal (2PN) WT and EphB-deficient mice
| DN1 | DN2 | DN3 | DN4 | |
|---|---|---|---|---|
| (a) E15·5 | ||||
| Wild type | 4·58 ± 1·92 | 5·45 ± 1·47 | 61·25 ± 5·80 | 28·25 ± 4·33 |
| EphB2−/− | 7·34 ± 2·33** | 7·00 ± 1·05** | 57·59 ± 3·07* | 27·45 ± 4·38 |
| EphB3−/− | 5·92 ± 1·31* | 6·85 ± 1·59* | 58·07 ± 3·06* | 28·97 ± 3·00 |
| EphB2/B3−/− | 8·57 ± 1·91*** | 8·90 ± 2·27*** | 53·39 ± 5·94*** | 28·67 ± 2·80 |
| (b) E17·5 | ||||
| Wild type | 2·11 ± 0·63 | 4·44 ± 1·42 | 62·57 ± 7·34 | 30·39 ± 5·54 |
| EphB2−/− | 2·15 ± 0·34 | 4·27 ± 0·54 | 75·38 ± 5·17*** | 17·38 ± 5·90*** |
| EphB3−/− | 2·76 ± 0·60 | 4·94 ± 0·98 | 71·90 ± 4·38*** | 20·01 ± 5·79*** |
| EphB2/B3−/− | 1·99 ± 0·71 | 4·39 ± 1·15 | 74·14 ± 4·11** | 18·97 ± 3·38** |
| (c) 2PN | ||||
| Wild type | 4·99 ± 0·60 | 10·12 ± 1·12 | 58·79 ± 0·64 | 25·62 ± 1·40 |
| EphB2−/− | 9·84 ± 1·63** | 10·99 ± 2·91 | 65·45 ± 4·21** | 10·78 ± 3·30** |
| EphB3−/− | 6·81 ± 1·06* | 9·48 ± 2·00 | 69·89 ± 4·46** | 12·23 ± 4·00** |
| EphB2/B3−/− | 7·82 ± 1·81* | 10·24 ± 1·81 | 64·42 ± 2·59** | 15·32 ± 4·47* |
Showed data are means ± standard deviations.
P < 0·05
P < 0·01 and
P < 0·005.
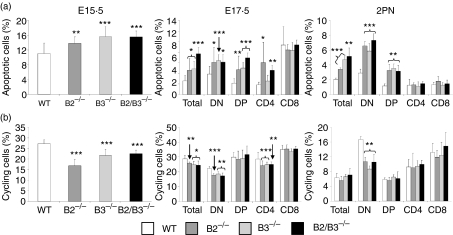
In correlation with the lower number of thymic cells observed in both fetal and neonatal thymuses of EphB-deficient mice, increased percentages of apoptotic thymocytes occurred as early as the 15·5-day fetal life (Fig. 8a). At that developmental stage, most thymocytes were DN cells that suffered increased apoptosis in the mutant mice. At 17·5 days of fetal life and in 2 days postnatal mice, increased apoptosis mainly affected DN cells but also DP thymocytes (Fig. 8a), as observed in the adult deficient thymuses. In addition, the percentage of total cycling cells significantly decreased in the fetal, but not in the neonatal thymuses of EphB-deficient mice (Fig. 8b). In the three stages studied, the population most affected by this reduction of cycling cell number was the DN cell compartment that even significantly decreased in the postnatal mutant mice (Fig. 8b).
Figure 8.
Both fetal and neonatal EphB-deficient mice showed variations in the proportions of apoptotic cells and cycling thymocytes. (a) Increased percentage of total apoptotic cells occurred in the fetal and neonatal thymuses of all analyzed stages. In both E17.5 and 2PN thymuses increased proportions of total apoptotic cells correlated with those largely observed in both DN and DP thymocytes. (b) Decreased total cycling cells were also observed in EphB-deficient mice, and in general DN cells were the most affected thymic cell compartment.
These results suggested, therefore, that the alterations observed in the cellularity and T-cell maturation of adult EphB-deficient mice originated early in the ontogeny of thymus and gradually increase to result in the adult thymus phenotype described.
Discussion
Eph and ephrin, molecules involved in numerous physiological processes including histo- and organogenesis, cell positioning and cell attraction/repulsion phenomena, etc.1 have been, however, little investigated in the immune system. The present results confirm and extend previous studies by us2,5,6 and other authors11,30,35 suggesting their involvement in thymus biology.
Our study shows a wide expression of EphB2 and EphB3 as well as ephrinB1 and ephrinB2 in the murine thymus. By RT–PCR, the four molecules are detected in the main thymocyte subsets as well as in the TEC of adult and fetal mice. Few studies have focused on the expression of Eph/ephrinB family in the thymus and these have largely centred on EphB68 and ephrinB.4,11 Previous reports had demonstrated that ephrinB2 was largely present in the cortex where both DP and SP cells in transition to the medulla and TEC predominate and, to a lesser extent, in the cortico-medullary border.8,36 By flow cytometry, Yu and colleagues have detected both ephrinB1 and ephrinB3 in all thymocyte subsets, especially in SP CD4− CD8+ thymocytes and DP cells.11,37 These same authors, by using both ephrinB1–Fc and ephrinB3–Fc fusion proteins, detected EphB expression in all thymocytes except DN cells.11 On the contrary, Shimoyama and collaborators8 have reported interaction between ephrinB1–Fc and fetal DN thymocytes.
By immunofluorescence, our topological study on the distribution of these molecules throughout the thymus demonstrates, confirming the RT–PCR results, that both EphB2/B3 and ephrinB1/B2 are expressed through all thymic compartments. In agreement, a broad expression pattern for ephrinB2 has been described in the thymus.9 However, other authors, using in situ hybridization, reported the expression of both ephrinB111 and ephrinB236 largely in the thymic cortex rather than in the medulla. Moreover,9 Cole and colleagues restricted ephrinB1 expression to the thymic subcapsular region. The different sensitivity of the techniques used could explain the different expression patterns reported. In any case, it is important to remark that this reported expression pattern for Eph/ephrinB family is different to that previously reported for the family A by us2 and other authors,3 suggesting different functions for the two families A and B in the thymus.
On the other hand, this homogeneous expression pattern that affects thymocytes but also cortical and medullary epithelial cells, could suggest that EphB2 and EphB3 play redundant functions in the thymus. However, our results indicate that the absence of EphB2 is not made up by EphB3 and vice versa. In this regard, our results also demonstrate that the absence of EphB2 and/or EphB3 does not affect the expression pattern of their ligands, ephrinB1 and ephrinB2, as we had previously observed in EphA4-deficient mice.6 Accordingly, the observed thymic phenotype of EphB-deficient mice is caused by the lack of EphB2 and/or EphB3 rather than alterations in the presence of their ligands.
The existence of a thymus phenotype in EphB-deficient mice confirms a role for Eph/ephrin family B in the functions of this key primary lymphoid organ, extending our own previous in vitro2,5 and in vivo studies6 on the relevance of Eph/ephrins family A in thymus functionality. Other studies, however, were unable to find evidence of this role for these molecules in the thymus gland. Normal cell content and unaltered thymic histology have been described in the thymus of both EphB29 and EphB6 knockout (KO) mice, although in these last ones peripheral T-cells show compromised functions7,8 and EphB6 transgenic mice exhibit breakdown of the thymic cortico-medullary border. Other authors have reported, however, that ephrinB1, one of the main ligands of EphB2, is critical for T-cell development.10
On the other hand, the phenotype of these deficient thymuses is really remarkable. Together with an important decreased cell content that correlates well with increased apoptosis and reduced numbers of cycling cells, they exhibit significant accumulation of the percentage of DN cells and decreased numbers of all thymocyte subsets defined by CD4/CD8 expression, largely DP and SP cells, but unchanged relative proportions of both DP and SP thymocytes. In addition, although the percentage of TCRγδ cells increases, the absolute numbers of this thymic cell subset do not undergo significant variations with respect to control values. On the other hand, independently of the number of immigrant precursors that arrive at the thymus, there are no differences in the numbers of both DN1 and DN2 cells between EphB-deficient and WT mice, but from the DN2 stage onward the thymocyte number begins to decrease. In these last stages of the DN compartment, thymocytes synthesize a pre-TCR that undergoes the so-called β-selection, necessary for expanding the thymocyte number and reaching the DP compartment.38 Accordingly, β-selection could be affected by the lack of EphB2 and/or EphB3 resulting in a low production of thymocytes capable of progressing to the DP compartment. In this respect, our results also demonstrate that, although all T-cell subsets show a reduced number of cycling cells, only the DN cells exhibit statistically significant differences compared with control values. Direct relationships between Eph/ephrin and β-selection have not been described although we recently demonstrate that Eph/ephrinB affects DP selection.5 Furthermore, Shc, an adaptor molecule involved in pre-TCR signalling,39 is associated with Eph and ephrin in other cell types.40,41
The decreased cellularity of EphB-deficient mice is also related to an increased percentage of apoptotic cells, mainly affecting both DN and DP cell compartments. In this respect, the reduced numbers of SP thymocytes could be related to the low number of DP cells available to undergo the final intrathymic maturation and/or to unknown defects in DP-SP transition. We recently reported, by using reaggregated thymic organ cultures established by fetal TEC and DP, that ephrinB1-Fc proteins were able to disorganize in vitro the three-dimensional epithelial network and to alter the thymocyte interactions. In addition, in an in vitro model, Eph/ephrinB–Fc treatment also decreased the formation of cell conjugates formed by DP and TEC as well as the TCR-dependent signalling between both cell types.5 Increasing evidence relates Eph/ephrin with the survival, proliferation and death of numerous cell types, including thymocytes and peripheral T lymphocytes. In vivo and in vitro treatment with different Eph/ephrin stimulates the proliferation of neural precursors42–44 whereas ephrinA2-deficient mice show decreased proliferation of neural progenitors.44 In the immune system the results are controversial. Reduced numbers of thymocytes have been described in EphB6 transgenic mice.9 EphB6-deficient mice did not show, however, changes in thymic cellularity8 but did present altered functionality of peripheral T cells.7 On the contrary, we demonstrated that in vitro addition of different EphA–Fc fusion proteins to rat FTOC results in decreased numbers of the yielded thymocytes, largely of DP cells2 and the supply of either EphB2–Fc or ephrinB1–Fc fusion proteins to mouse FTOC decreases the numbers of both DP and SP thymocytes, in correlation with increased apoptosis.5 Freywald and colleagues45 demonstrated, however, that the stimulation of murine thymocytes with ephrinA1-Fc reduced TCR-mediated apoptosis. Previously, this same group had reported that cross-linking of EphB6 with anti-ephrinB1 antibody protected CD3+ thymocytes from anti-CD3 antibody-induced apoptosis.30 Yu and collaborators11 have also described that ephrinB1-Fc costimulation protects from anti-CD3 antibody-induced apoptosis of thymocytes. These contradictory results could be, however, understood considering that recent data demonstrate that the Eph/ephrinB-dependent modulation of anti-CD3 antibody-induced apoptosis of DP thymocytes is a process dependent on concentration.5
Numbers of T lymphocytes diminish at the periphery, largely in mesenteric lymph nodes and peripheral blood, but not in spleen, with higher differences in the double mutant lymph nodes than in EphB2 or EphB3 KO mice. No significant differences were found in the proportions of these cells defined by CD4/CD8 expression. Decreased numbers of peripheral T cells without variations in the proportions of their subsets could reflect lower numbers, but no percentages, of SP thymocytes produced in the EphB-deficient thymuses, as compared to control ones, that migrate to the periphery colonizing the secondary lymphoid organs. On the other hand, the different distribution of T cells in lymph nodes, spleen and peripheral blood would suggest an involvement of chemokines and their receptors, which governs the specific migration toward and between different lymphoid tissues, a process in which EphB2, EphB3, ephrinB1 and ephrinB2 have been implicated.24,31,46
One of the most remarkable features of the phenotype of EphB-deficient thymuses is that, despite the profound epithelial disorganization occurring,5 there are no important changes in the percentage of thymocyte subsets, except for the accumulation of DN cells. On the contrary, EphA4-deficient mice exhibit a collapse of thymic cortical epithelium that provokes the blockade of T-cell differentiation with a pronounced drop in the proportion of DP thymocytes.6 Nevertheless, in other experimental models, thymuses that exhibit important alterations of the thymic stroma do not show a relevant thymocyte phenotype. For instance, adult mice that do not express signal transducer and activator of transcription-3 in the TEC but do express it in the thymocytes exhibit, like the EphB-deficient mice, profound alterations in the thymic epithelial meshwork and decreased numbers of thymocytes.47 Altered thymic epithelium with increased numbers of K5 positive cells, as observed in EphB-deficient mice5 and occurs in FGFR2IIIb-deficient mice allows T-cell differentiation,48 and Krm1 KO mice, which exhibit similar modifications of the thymic epithelium to those observed in our mice, also show normal proportions of T-cell subsets.49 Although we do not as yet have an answer for these phenomena, it is possible, as previously pointed out for other deficient mice,48,49 that the maintenance of some unaltered epithelial areas in the deficient thymuses is sufficient to support a relatively normal T-cell differentiation.
Regarding the origin of the phenotype observed, our results demonstrate that this is not an exclusive feature of adult mutants. On the contrary, it already occurs in neonatal and 15·5-day fetal mice, suggesting that EphB2 and EphB3 are important not only for the adult thymus but also for its histogenesis. The adult phenotype could, therefore, be a consequence of the gradual accumulation of defects affecting, apart from the maturation of TEC (J. García-Ceca, E. Jiménez, D. Alfaro, T. Cejalvo, J. J. Muñoz, A. Zapata, unpublished data), the numbers of DN cells capable of reaching the DP compartment, as well as the numbers of immature thymocytes that undergo apoptosis or division.
Acknowledgments
We would like to thank the Microscopy and Cytometry Centre of Complutense University of Madrid for the use of their facilities and technical assistance. We also thank the Developmental Studies Hybridoma Bank of Iowa University for supplying the anti-K8 keratin antibody. This work was supported by grants BFU2004-03132 and RD06/0010/003 from the Spanish Ministry of Education and Culture and S-BIO-0204-2006 from the Madrid Government.
Abbreviations
- DN
double negative
- DP
double positive
- FTOC
fetal thymic organ culture
- SP
single positive
- TEC
thymic epithelial cell
References
- 1.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 2.Munoz JJ, Alonso CL, Sacedon R, Crompton T, Vicente A, Jimenez E, Varas A, Zapata AG. Expression and function of the Eph A receptors and their ligands ephrins A in the rat thymus. J Immunol. 2002;169:177–84. doi: 10.4049/jimmunol.169.1.177. [DOI] [PubMed] [Google Scholar]
- 3.Vergara-Silva A, Schaefer KL, Berg LJ. Compartmentalized Eph receptor and ephrin expression in the thymus. Mech Dev. 2002;119(Suppl. 1):S225–9. doi: 10.1016/s0925-4773(03)00121-7. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Luo H. Recent advances on T-cell regulation by receptor tyrosine kinases. Curr Opin Hematol. 2005;12:292–7. doi: 10.1097/01.moh.0000166497.26397.9f. [DOI] [PubMed] [Google Scholar]
- 5.Alfaro D, Garcia-Ceca JJ, Cejalvo T, Jimenez E, Jenkinson EJ, Anderson G, Munoz JJ, Zapata A. EphrinB1-EphB signaling regulates thymocyte–epithelium interactions involved in functional T cell development. Eur J Immunol. 2007;37:2596–605. doi: 10.1002/eji.200737097. [DOI] [PubMed] [Google Scholar]
- 6.Munoz JJ, Alfaro D, Garcia-Ceca J, Alonso CL, Jimenez E, Zapata A. Thymic alterations in EphA4-deficient mice. J Immunol. 2006;177:804–13. doi: 10.4049/jimmunol.177.2.804. [DOI] [PubMed] [Google Scholar]
- 7.Luo H, Yu G, Tremblay J, Wu J. EphB6-null mutation results in compromised T cell function. J Clin Invest. 2004;114:1762–73. doi: 10.1172/JCI21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimoyama M, Matsuoka H, Nagata A, et al. Developmental expression of EphB6 in the thymus: lessons from EphB6 knockout mice. Biochem Biophys Res Commun. 2002;298:87–94. doi: 10.1016/s0006-291x(02)02399-9. [DOI] [PubMed] [Google Scholar]
- 9.Coles MC, Adams R, Adams S, Roderick K, Norton T, Wilkinson D, Kioussis D. The role of Eph receptors and ephrins ligands in T-cell development in the thymus. Clin Invest Med; 12th Int Congress of Immunology and 4th Annual Conference of FOCIS, Montreal, Canada, July 18–23.2004. [Google Scholar]
- 10.Yu G, Luo H, Wu Y, Wu J. EphrinB1 is essential in T-cell–T-cell co-operation during T-cell activation. J Biol Chem. 2004;279:55531–9. doi: 10.1074/jbc.M410814200. [DOI] [PubMed] [Google Scholar]
- 11.Yu G, Mao J, Wu Y, Luo H, Wu J. Ephrin-B1 is critical in T-cell development. J Biol Chem. 2006;281:10222–9. doi: 10.1074/jbc.M510320200. [DOI] [PubMed] [Google Scholar]
- 12.Birgbauer E, Cowan CA, Sretavan DW, Henkemeyer M. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development. 2000;127:1231–41. doi: 10.1242/dev.127.6.1231. [DOI] [PubMed] [Google Scholar]
- 13.Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 14.Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J. 1996;15:6035–49. [PMC free article] [PubMed] [Google Scholar]
- 15.Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–63. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 16.Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–90. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Foo SS, Turner CJ, Adams S, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–73. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg J, Genander M, Halford MM, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–63. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Batlle E, Bacani J, Begthel H, et al. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–30. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 20.Clevers H, Batlle E. EphB/EphrinB receptors and Wnt signaling in colorectal cancer. Cancer Res. 2006;66:2–5. doi: 10.1158/0008-5472.CAN-05-3849. [DOI] [PubMed] [Google Scholar]
- 21.Guo DL, Zhang J, Yuen ST, et al. Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumours. Carcinogenesis. 2006;27:454–64. doi: 10.1093/carcin/bgi259. [DOI] [PubMed] [Google Scholar]
- 22.Hafner C, Meyer S, Langmann T, et al. Ephrin-B2 is differentially expressed in the intestinal epithelium in Crohn's disease and contributes to accelerated epithelial wound healing in vitro. World J Gastroenterol. 2005;11:4024–31. doi: 10.3748/wjg.v11.i26.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafner C, Schmitz G, Meyer S, et al. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–9. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- 24.Sharfe N, Freywald A, Toro A, Dadi H, Roifman C. Ephrin stimulation modulates T cell chemotaxis. Eur J Immunol. 2002;32:3745–55. doi: 10.1002/1521-4141(200212)32:12<3745::AID-IMMU3745>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Brambilla R, Bruckner K, Orioli D, Bergemann AD, Flanagan JG, Klein R. Similarities and differences in the way transmembrane-type ligands interact with the Elk subclass of Eph receptors. Mol Cell Neurosci. 1996;8:199–209. doi: 10.1006/mcne.1996.0057. [DOI] [PubMed] [Google Scholar]
- 26.Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–9. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- 27.Gale NW, Holland SJ, Valenzuela DM, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 28.Luo H, Wan X, Wu Y, Wu J. Cross-linking of EphB6 resulting in signal transduction and apoptosis in Jurkat cells. J Immunol. 2001;167:1362–70. doi: 10.4049/jimmunol.167.3.1362. [DOI] [PubMed] [Google Scholar]
- 29.Shimoyama M, Matsuoka H, Tamekane A, et al. T-cell-specific expression of kinase-defective Eph-family receptor protein, EphB6 in normal as well as transformed hematopoietic cells. Growth Factors. 2000;18:63–78. doi: 10.3109/08977190009003234. [DOI] [PubMed] [Google Scholar]
- 30.Freywald A, Sharfe N, Rashotte C, Grunberger T, Roifman CM. The EphB6 receptor inhibits JNK activation in T lymphocytes and modulates T cell receptor-mediated responses. J Biol Chem. 2003;278:10150–6. doi: 10.1074/jbc.M208179200. [DOI] [PubMed] [Google Scholar]
- 31.Aasheim HC, Delabie J, Finne EF. Ephrin-A1 binding to CD4+ T lymphocytes stimulates migration and induces tyrosine phosphorylation of PYK2. Blood. 2005;105:2869–76. doi: 10.1182/blood-2004-08-2981. [DOI] [PubMed] [Google Scholar]
- 32.Smith FM, Vearing C, Lackmann M, et al. Dissecting the EphA3/Ephrin-A5 interactions using a novel functional mutagenesis screen. J Biol Chem. 2004;279:9522–31. doi: 10.1074/jbc.M309326200. [DOI] [PubMed] [Google Scholar]
- 33.Gray DH, Chidgey AP, Boyd RL. Analysis of thymic stromal cell populations using flow cytometry. J Immunol Methods. 2002;260:15–28. doi: 10.1016/s0022-1759(01)00493-8. [DOI] [PubMed] [Google Scholar]
- 34.Laurent J, Bosco N, Marche PN, Ceredig R. New insights into the proliferation and differentiation of early mouse thymocytes. Int Immunol. 2004;16:1069–80. doi: 10.1093/intimm/dxh108. [DOI] [PubMed] [Google Scholar]
- 35.Wu XW, Li M. The Eph receptors and ephrins in synaptic plasticity. Sheng Li Ke Xue Jin Zhan. 2005;36:259–61. [PubMed] [Google Scholar]
- 36.Yu G, Luo H, Wu Y, Wu J. Ephrin B2 induces T cell costimulation. J Immunol. 2003;171:106–14. doi: 10.4049/jimmunol.171.1.106. [DOI] [PubMed] [Google Scholar]
- 37.Yu G, Luo H, Wu Y, Wu J. Mouse ephrinB3 augments T-cell signaling and responses to T-cell receptor ligation. J Biol Chem. 2003;278:47209–16. doi: 10.1074/jbc.M306659200. [DOI] [PubMed] [Google Scholar]
- 38.von Boehmer H, Aifantis I, Azogui O, Feinberg J, Saint-Ruf C, Zober C, Garcia C, Buer J. Crucial function of the pre-T-cell receptor (TCR) in TCR beta selection, TCR beta allelic exclusion and alpha beta versus gamma delta lineage commitment. Immunol Rev. 1998;165:111–9. doi: 10.1111/j.1600-065x.1998.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Camerini V, Bender TP, Ravichandran KS. A nonredundant role for the adapter protein Shc in thymic T cell development. Nat Immunol. 2002;3:749–55. doi: 10.1038/ni820. [DOI] [PubMed] [Google Scholar]
- 40.Pratt RL, Kinch MS. Activation of the EphA2 tyrosine kinase stimulates the MAP/ERK kinase signaling cascade. Oncogene. 2002;21:7690–9. doi: 10.1038/sj.onc.1205758. [DOI] [PubMed] [Google Scholar]
- 41.Vindis C, Cerretti DP, Daniel TO, Huynh-Do U. EphB1 recruits c-Src and p52Shc to activate MAPK/ERK and promote chemotaxis. J Cell Biol. 2003;162:661–71. doi: 10.1083/jcb.200302073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aoki M, Yamashita T, Tohyama M. EphA receptors direct the differentiation of mammalian neural precursor cells through a mitogen-activated protein kinase-dependent pathway. J Biol Chem. 2004;279:32643–50. doi: 10.1074/jbc.M313247200. [DOI] [PubMed] [Google Scholar]
- 43.Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–7. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 44.Holmberg J, Armulik A, Senti KA, et al. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–71. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freywald A, Sharfe N, Miller CD, Rashotte C, Roifman CM. EphA receptors inhibit anti-CD3-induced apoptosis in thymocytes. J Immunol. 2006;176:4066–74. doi: 10.4049/jimmunol.176.7.4066. [DOI] [PubMed] [Google Scholar]
- 46.Smith LM, Walsh PT, Rudiger T, Cotter TG, Mc Carthy TV, Marx A, O’Connor R. EphA3 is induced by CD28 and IGF-1 and regulates cell adhesion. Exp Cell Res. 2004;292:295–303. doi: 10.1016/j.yexcr.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 47.Sano S, Takahama Y, Sugawara T, et al. Stat3 in thymic epithelial cells is essential for postnatal maintenance of thymic architecture and thymocyte survival. Immunity. 2001;15:261–73. doi: 10.1016/s1074-7613(01)00180-7. [DOI] [PubMed] [Google Scholar]
- 48.Revest JM, Suniara RK, Kerr K, Owen JJ, Dickson C. Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J Immunol. 2001;167:1954–61. doi: 10.4049/jimmunol.167.4.1954. [DOI] [PubMed] [Google Scholar]
- 49.Osada M, Ito E, Fermin HA, Vazquez-Cintron E, Venkatesh T, Friedel RH, Pezzano M. The Wnt signaling antagonist Kremen1 is required for development of thymic architecture. Clin Dev Immunol. 2006;13:299–319. doi: 10.1080/17402520600935097. [DOI] [PMC free article] [PubMed] [Google Scholar]