Abstract
Increased intima-media thickness (IMT) and pulse wave velocity (PWV) are noninvasive markers of early arterial wall alteration and are more widely used in adult clinical research. We investigated whether IMT and PWV are useful predictors of cardiovascular risk in hypertensive adolescents. Fifteen hypertensive adolescents (13-18 yr old, systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg) and seventeen normotensive subjects were included. Height, weight, obesity index, body mass index (BMI), and fat distribution were obtained from each group. Serum lipid, insulin, vitamine B12, folate, renin, aldosterone, angiotensin-converting enzyme (ACE), and homocysteine levels were compared. The carotid IMT and PWV were measured. Arterial wall compliance and distensibility were calculated with the equation. High systolic blood pressure significantly correlated with height, weight, BMI, obesity index, arm circumference, fat mass, and fat distribution. Hypertensive adolescents had significantly greater cIMT (carotid intima-media thickness) and lower elastic properties such as cross-sectional compliance and distensibility of the carotid artery. The carotid IMT significantly correlated with brachial-ankle PWV. In conclusion, the measurement of carotid IMT and brachial-ankle PWV might be useful to predict the development of atherosclerosis in hypertensive adolescents.
Keywords: Carotid Intima-Media Thickness (cIMT), Pulse Wave Velocity (PWV), Hypertension, Atherosclerosis, Adolescent
INTRODUCTION
The disease process of atherosclerosis begins in childhood. In an autopsy study, fatty steaks were found in the aortas of children older than 3 yr and in the coronary arteries by adolescence (1). Although cardiovascular morbidity is rare during children as a result of hypertension, many researches suggest that sequelae of hypertension such as left ventricular hypertrophy and diastolic ventricular dysfunction begin years of decades before the onset of coronary artery disease and stroke.
In adults, non-invasive ultrasonographic assessment of large arteries and increased intima-media thickness (IMT) of the carotid artery (cIMT) have been shown to correlate with cardiovascular risk in hypertensive patients (2, 3). Increased cIMT has also been reported in diabetic children (4), children with familial hypercholesterolemia (5), growth hormone deficiency (6), William syndrome (7), and Kawasaki disease (8), compared with normal controls. However, there are few studies on the correlation between cIMT and cardiovascular risk in hypertensive children. Although the ultrasound method for measuring cIMT does not allow for differentiation of intimal atherosclerosis from medial hypertrophy due to pressure effects, increased cIMT is well established as a marker for cardiovascular risk in adults. Recently, studies suggested that IMT represents an early manifestation of vasculopathy in children.
Pulse wave velocity (PWV), another non-invasive method measuring arterial stiffness, has been noted as a useful method of evaluating the severity of atherosclerosis in adults (9). Recently, a novel device that measures brachial-ankle PWV (ba PWV) was developed. The validity and reproducibility of baPWV measurements were reported in adults and the method seemed to be an acceptable marker reflecting vascular damage.
A relationship between aortic stiffness and hypertension (10) has also been noted in children. Only limited data on PWV in hypertensive children have been reported.
The purpose of this study was to investigate whether IMT and PWV are useful diagnostic markers in predicting early atherosclerosis in adolescents with essential hypertension.
MATERIALS AND METHODS
Patients
Fifteen hypertensive patients (14 male and 1 female) and 17 normotensive subjects (12 male and 5 females), aged between 16-18 yr, were involved in the study.
The hypertensive adolescents were recruited from the middle and high school students screened for hypertension. They were newly diagnosed patients who were diagnosed as essential hypertension subsequently. We only included whose systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Elevated blood pressure was confirmed in a pediatric clinic by averaging the 3 blood pressure measurements made after 5 min of rest with an oscillometric monitor.
Informed consent was obtained from the parents and study participants.
Bioelectrical impedance analysis
Measurements were made with a segmental bioelectrical impedance analyzer, using eight tactile electrodes according to the manufacturer's instructions (InBody 3.0; Biospace, Seoul, Korea). A pair of electrodes was in placed on the surface of each hand and foot and hand. These electrodes were connected to current and voltage terminals from an impedance meter via electronic on/off switches, which were regulated by a microprocessor. The target segment could be measured selectively.
Intima-media thickness of common carotid artery
The same investigator performed non-invasive measurements on the carotid artery using a real-time B-mode ultrasound imager (iU22, intelligent Ultrasound System; Philips, Amsterdam, Netherlands) and a probe of 12.5 MHz. For all subjects, the IMT and lumen diameter were measured in the same arterial segment.
The measurements were performed within 1 cm above the bifurcation of the right common carotid artery. The IMT of artery was defined as the distance between two parallel echogenic (bright) lines of the vessel in the sonography. The first line near the lumen is the intimal-luminal interface, and the second one, close to the adventitia, is produced by the collagen containing the upper layer of the tunica adventitia. IMT, lumen diastolic (dD), and systolic diameters (sD) were measured in the anteroposterior projection with the patient lying supine, after at least 30 min of recombent position.
The measurements were substituted for calculation according to the following formulae described by Aggoun et al. (11).
Lumen cross-sectional area=πdD2/4
Wall cross-sectional area=π(dD/2+IMT)2-π(dD/2)2.
Cross-sectional compliance=[π(sD2-dD2)]/4△P (mm2.mmHg-1)
Cross-sectional distensibility=(sD2-dD2)/(dD2△P)(mmHg-1.10-2)
△P: pulse pressure
Pulse wave velocity
baPWV was measured in supine position by VP-1000 (Colin Co., Komaki, Japan). Using volume plethysmographic technique, PWV, ABI (Ankle brachial index, the ratio of systolic blood pressure in the ankle to that in the brachial artery), blood pressure, electrocardiography and pulse were obtained simultaneously. Sphygmomanometer cuffs were wrapped on both the brachia and ankles, electrocardiogram electrodes were placed on both wrists, and a microphone was placed on the left edge of the sternum. As the pulse wave contours in the four extremities were recorded, the cuffs inflated and deflated automatically. Pulse transit time between the brachial and ankle regions was computed from these pulse volume recordings. baPWV was determined from the pulse transit time and the distance between these two segments. The distance of each segment was calculated automatically, based on the height of the subjects.
Lipid profile
Blood samples were taken from venous puncture after midnight fasting. The following laboratory studies were performed in all subjects and controls: fasting lipids levels [total, low-density lipoprotein (LDL)-, high-density lipoprotein (HDL)-cholesterol, and triglycerides], plasma homocysteine, insulin, aldosterone, renin and angiotensin converting enzyme (ACE).
The plasma cholesterol concentration was measured with an enzymatic colorimetry (ADVIA 1650; Bayer, Pennsylvania, U.S.A.) and triglyceride concentrations were measured with an enzymatic method.
Homocysteine, insulin, Vit B12 and folate
Plasma homocysteine was measured by automated Bayer ADVIA Centaur chemiluminescent Hcy assay. Insulin was determined using radio-immunoassay (RIA) commercially available kit by Biosource, Belgium with Gamma counter (Hewlett Packard, California, U.S.A.).
Plasma concentrations of vitamin B12 and folate were quantified with a radioassay Gamma Counter (Packard, California, U.S.A.).
Renin, aldosterone and ACE
Plasma renin and aldosterone were measured with COBRA-II Gamma counter (Packard, California, U.S.A.). Blood samples for renin concentration were taken after 30 min supine rest and measured directly by an improved immunoradiometric assay.
Statistical analysis
All statistical analyses were done with SAS system (Windows version 11.0).
Descriptive statistics were presented as percentages, means, and standard deviations. Univariate analyses for group comparisons of continuous variables were performed using student t-test.
The correlations among continuous variables were determined using the Pearson correlation coefficient. A p value less than 0.05 was regarded as significant.
RESULTS
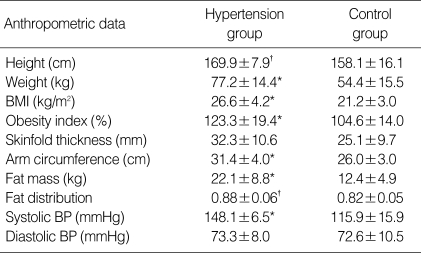
The majority of subjects in the study were male (87.5%). Sixty percent of hypertensive adolescents and 17% of control were obese, defined as BMI ≥95th percentile for age and gender. Hypertensive subjects had significantly higher weight, height, BMI, obesity index, arm circumference, fat mass, fat distribution and systolic blood pressure as shown in Table1.
Table 1.
Anthropometric data of study group
*p<0.01 Significantly different from control group; †p<0.05 Significantly different from control group.
BMI, body mass index; BP, blood pressure.
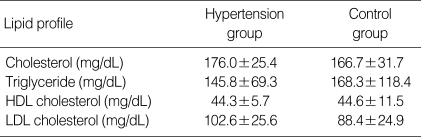
Among 15 hypertensive adolescents, 12 (80%) had isolated systolic hypertension, and 3 (20%) had both systolic and diastolic hypertension. There was no significant difference in the mean of serum lipid level between two groups (Table 2).
Table 2.
Comparison of lipid profile in hypertension group and control group
*p<0.05 Significantly different from control group.
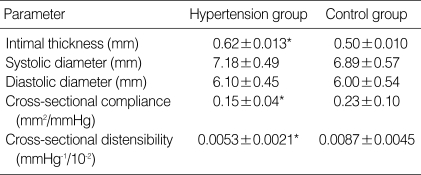
Hypertensive adolescents had significantly greater values of cIMT (0.62±0.013 mm vs. 0.50±0.010 mm) (p<0.05) than normotensive children. Elastic properties such as cross-sectional compliance (0.15±0.04 mm2/mmHg vs. 0.23± 0.10 mm2/mmHg) and distensibility (0.0053±0.0021 mmHg-1/10-2 vs. 0.0087±0.0045 mmHg-1/10-2) of the carotid artery were also significantly different between hypertensive and normotensive group (p<0.05) (Table 3).
Table 3.
Geometrical analysis of the common carotid artery in hypertension group and control group
*p<0.05 Significantly different from control group.
Several biochemical indices which are known to be related to hypertension and coronary disease were compared. Serum homocysteine, folate, insulin and vitamin B12 were measured. Only vitamin B12 level showed significant decrease (551.5±251.7 pg/mL vs. 859.8±454.4 pg/mL ) in hypertensive group (p<0.05) (Table 4).
Table 4.
Comparison of insulin, homocysteine, vitamine B12, and folate in hypertension group and control group
*p<0.05 Significantly different from control group.
Renin, aldosterone and ACE levels were also measured and no significant difference was observed in our study (Table 5).
Table 5.
Comparison of renin, aldosterone, and angiotensin converting enzyme in hypertension group and control group
*p<0.05 significantly different from control group.
ACE, angiotensin converting enzyme.
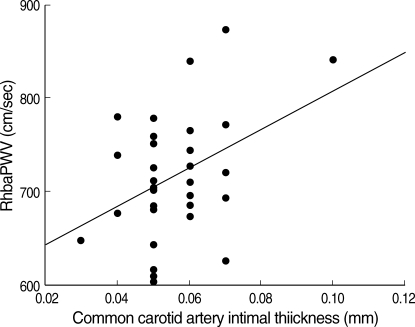
PWV were measured separately for the comparison. Right heart brachial-ankle pulse wave velocity (RhbaPWV) and left heart brachial-ankle pulse wave velocity (LhbaPWV) of hypertensive group were 745.9±63.2 cm/sec and 759.2±60.8 cm/sec respectively, which were significantly increased compare to normal control (p<0.05) whose average values were 690.6±56.0 cm/sec and 702.6±51.5 cm/sec for right and left side of the body (Table 6). There was significant correlation between the hbaPWV and IMT (Fig. 1, 2).
Table 6.
Comparison of pulse wave velocities in hypertension group and control group
*p<0.05 significantly different from control group.
RhbaPWV, Right heart brachial-ankle pulse wave velocity; LhbaPWV, Left heart brachial-ankle pulse wave velocity.
Fig. 1.
There was a significant linear correlation between common carotid artery IMT and RhbaPWV. The equation of slope is Y=7.658E-0.5X+6.434E-0.4 (r=0.396, p<0.05).
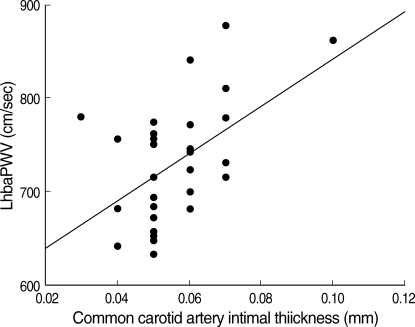
Fig. 2.
There was a significant linear correlation between common carotid artery IMT and LhbaPWV. The equation of slope is Y=1.030E-0.4X -2.0E-0.2 (r=0.51, p<0.05).
DISCUSSION
Hypertension is one of the leading factors for the development of atherosclerosis. Although serious morbidity occurs rarely, essential hypertension starts to develop in childhood. Some end organ complications are also reported in children. Left ventricular hypertrophy and retinal hypertensive angiopathy are observed (12). Impaired left ventricular relaxation, left ventricular remodeling, and a high prevalence of left ventricular hypertrophy are reported as a result of the diastolic dysfunction of hypertensive children (13).
Noninvasive tests such as carotid artery B-mode ultrasound, PWV, ankle brachial blood pressure ratios, carotid artery duplex scanning, electron beam-computed tomography, ultrasound-based endothelial function studies and magnetic resonance imaging techniques offer the potential method for measuring and monitoring atherosclerosis.
IMT of large artery walls, especially carotid, can be assessed by B-mode ultrasound in a relatively simple way and represents a safe, inexpensive, precise and reproducible measure (14). Recent studies have suggested that preclinical hypertensive sequelae may also be detected by the use of vascular ultrasonography. In adults, increased IMT of the carotid artery has been shown to indicate the presence of atherosclerosis and predict cardiovascular morbidity and mortality (15).
Since carotid IMT is a marker of early arterial wall change, including atherosclerosis and/or vascular hypertrophy, its detection by B-mode ultrasonography might participate in the diagnosis of high cardiovascular risk and in the decision to treat aggressively those patients at risk with drug treatments (16). Studies in adults with hypertension have shown that IMT in common carotid arteries correlates with cardiovascular risk and the severity of hypertension (17). Before proposing the routine measurement of IMT in clinical practice several limitations have to be overcome such as the standardization of methods of measurement including the site and the analysis of the measurement and a precise definition of the threshold of IMT (18).
Increased IMT is regarded as one of the first signs of early atherosclerosis in adult. There are few reports that have indicated a correlation between cIMT and hypertension in children (19). Recently, Sorof et al. (20) reported significantly increased cIMT in hypertensive compared with healthy children.
We found that hypertension is accompanied by greater IMT and diminished elasticity of the carotid artery. Such changes are visible in childhood. In a recent study of Sorof et al. (20) carotid IMT was correlated with BMI, age, height, and weight in groups of healthy and hypertensive children. But significant correlation was not noted between carotid IMT and BMI or weight in this study. Mechanical properties of the arterial wall, including distensibility, compliance were also deranged in hypertensive children.
Some studies of hypertensive adults have reported an increased lumen diameter of the common carotid artery and an increase in the IMT to lumen ratio (21). Distensibility is inversely correlated with pulse pressure and this variable was significantly greater in the hypertensive group (12). In our study, decreased distensibility and compliance were noted in hypertensive adolescents. The reduction of artery wall distensibility and compliance of the elastic arteries in essential hypertension is still incompletely understood. But it is suggested by the increase in blood pressure or intrinsic changes in the wall.
Essential hypertension must last sufficiently long to cause remodeling of the arterial wall, which will further reduce compliance of the artery. The incremental elastic modulus of the artery is independent of the lumen diameters and reflects the elastic properties of the arterial wall. Increased IMT may be related not only to hypertension, but also to other factors damaging the vascular wall, such as homocysteine or lipids (12). However, in our study there was no significant change of serum lipid and or homocysteine levels in hypertensive children compared with control group. Renin, aldosteron, ACE level were not different between two groups except vitamin B12 level.
Recently, PWV has been noted as a simple, non-invasive, and automatic method for evaluating arterial stiffness, which is strongly associated with systemic atherosclerosis at various sites in the vascular tree (22, 23). Measurement of carotid-femoral PWV is recognized as an established method to evaluate the severity of atherosclerosis and predictor of cardiovascular mortality in adults. The recently published study by Dernellis and Panaretou provided evidence that increased aortic stiffness precedes hypertension (24). They suggested that the alteration in arterial function is present already in nonhypertensive subjects at risk of hypertension and it may contribute to the progression to hypertension in later life (24). BaPWV would be essential for the screening of atherosclerosis in children.
There are very few reports on the influence of age and gender on arterial stiffness or reference value in healthy children (25). Since arterial stiffness is greatly influenced by age and gender. It is certainly necessary to evaluate the influence of age and gender on baPWV. Further, it has been shown that, in adults, baPWV is strongly influenced by gender, and that baPWV is higher in men than in women (26). In our previous study, baPWV was higher in normotensive male adolescents compared with female adolescents (27). In our previous report, a positive correlation was found between the systolic, diastolic, average blood pressure and PWV (28). This is because blood pressure directly affects pulse waves, and structural changes due to chronic hypertension increase PWV, in addition, stiffness of arterial walls itself affects blood pressure. In this our study, the carotid IMT and baPWV were significantly higher in hypertensive adolescents compared with normal control. And also, the carotid IMT significantly correlated with baPWV in hypertensive adolescents.
In our result, we found that hypertension is accompanied by greater IMT and diminished elasticity and distensibility in childhood. The differences between hypertensive and healthy children were highly significant although values of IMT in our patients were much lower than those reported in adults. However, there are no data on any cut-off value of carotid IMT in childhood, as an index of increased risk of cardiovascular events in adolescents.
There were several limitations in this study. This study was a small scale investigation including only 15 adolescents as subjects. Since arterial stiffness is greatly influenced by age and gender, it is certainly necessary to evaluate the influence of age and gender on baPWV. However we did not evaluate arterial stiffness according to age and gender.
In conclusion, we report that in children with essential arterial hypertension there is evidence of subclinical arterial damage i.e. endothelial dysfunction, changes in thickness, distensibilitiy and compliance of arterial wall. This causes not only an increase in IMT of the arterial wall but also a significant decrease in the elastic properties of the artery. Our results suggest that carotid IMT and PWV may be useful for predicting coronary artery disease and other cardiovascular sequale in hypertensive children. A larger scale investigation should promise more reliable results about carotid IMT and PWV in hypertensive adolescents.
References
- 1.Newman WP, 3rd, Wattigney W, Berenson GS. Autopsy studies in United States children and adolescents. Relationship of risk factors to atherosclerotic lesions. Ann N Y Acad Sci. 1991;623:16–25. doi: 10.1111/j.1749-6632.1991.tb43715.x. [DOI] [PubMed] [Google Scholar]
- 2.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattingney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The bogalusa heart study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 3.Aminbakhsh A, Mancini GB. Carotid intima-media thickness measurements: what define an abnormality? A systematic review. Clin Invest Med. 1999;22:149–157. [PubMed] [Google Scholar]
- 4.Rosfors S, Hallerstam S, Jensen-Urstad K, Zetterling M, Carlstrom C. Relationship between intima-media thickness in the common carotid artery and atherosclerosis in the carotid bifurcation. Stroke. 1998;29:1378–1382. doi: 10.1161/01.str.29.7.1378. [DOI] [PubMed] [Google Scholar]
- 5.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stoke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 6.Capaldo B, Patti L, Oliviero U, Longobardi S, Pardo F, Vitale F, Fazio S, Di Rella F, Biondi D, Lombardi G, Sacca L. Increased arterial intima-media thickness in childhood-onset growth hormone deficiency. J Clin Endocrinol Metab. 1997;82:1378–1381. doi: 10.1210/jcem.82.5.3951. [DOI] [PubMed] [Google Scholar]
- 7.Sadler LS, Gingell R, Martin DJ. Carotid ultrasound examination in Williams syndrome. J Pediatr. 1998;132:354–356. doi: 10.1016/s0022-3476(98)70461-5. [DOI] [PubMed] [Google Scholar]
- 8.Noto N, Okada T, Yamasuge M, Taniguchi K, Karasawa K, Ayusawa M, Sumitomo N, Harada K. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics. 2001;107:1095–1099. doi: 10.1542/peds.107.5.1095. [DOI] [PubMed] [Google Scholar]
- 9.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 10.Laurent S, Girerd X, Mourad JJ, Lacolley P, Beck L, Boutouyrie P, Mignot JP, Safar M. Elastic modulus of the radial artery wall materials is not increased in patients with essential hypertension. Arterioscler Thromb. 1994;14:1223–1231. doi: 10.1161/01.atv.14.7.1223. [DOI] [PubMed] [Google Scholar]
- 11.Aggoun Y, Sidi D, Levy BI, Lyonnet S, Kachaner J, Bonnet D. Mechanical properties of the common carotid artery in Williams syndrome. Heart. 2000;84:290–293. doi: 10.1136/heart.84.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Litwin M, Trelewicz Z, Wawer Z, Antoniewicz J, Wierzbicka A, Rajszys P, Grenda R. Intima-media thickness and arterial elasticity in hypertensive children: controlled study. Pediatr Nephrol. 2004;19:767–774. doi: 10.1007/s00467-004-1480-6. [DOI] [PubMed] [Google Scholar]
- 13.Sorof JM, Alexandrov AV, Cardwell G, Portman RJ. Carotid artery intimal-medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics. 2003;111:61–66. doi: 10.1542/peds.111.1.61. [DOI] [PubMed] [Google Scholar]
- 14.Simon A, Gariepy J, Chironi G, Megnien JL, Levenson J. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20:159–169. doi: 10.1097/00004872-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA. 1990;264:3018–3024. doi: 10.1001/jama.1990.03450230054029. [DOI] [PubMed] [Google Scholar]
- 16.Greenland P, Abrams J, Aurigemma GP, Bond MG, Clark LT, Criqui MH, Crouse JR, 3rd, Friedman L, Fuster V, Herrington DM, Kuller LH, Ridker PM, Roberts WC, Stanford W, Stone N, Swan HJ, Taubert KA, Wexler L. Prevention Conference V: beyond secondary prevention identifying the high-risk patient for primary prevention: noninvasive tests of atherosclerotic burden; Writing Group III. Circulation. 2000;101:E16–E22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 17.Berenson GS, Srinvasan SR, Boa W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 18.Simon A, Gariepy J, Levenson J. Is there a clinical future for echographic measurement of intima media thickness? Presse Med. 1999;28:1021–1022. [PubMed] [Google Scholar]
- 19.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 20.Sorof JM, Alexandrov AV, Garami Z, Turner JL, Grafe RE, Lai D, Portman RJ. Carotid ultrasonography for detection of vascular abnormalities in hypertensive children. Pediatr Nephrol. 2003;18:1020–1024. doi: 10.1007/s00467-003-1187-0. [DOI] [PubMed] [Google Scholar]
- 21.Roman MJ, Saba PS, Pini RP, Spitzer M, Pickering TG, Rosen S, Alderman MH, Devereux RB. Parallel cardiac and vascular adaptation in hypertension. Circulation. 1992;86:1909–1918. doi: 10.1161/01.cir.86.6.1909. [DOI] [PubMed] [Google Scholar]
- 22.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 23.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle wave velocity measurement. Hypertens Res. 2002;25:359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 24.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45:426–431. doi: 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- 25.Cheung YF, Brogan PA, Pilla CB, Dillon MJ, Redington AN. Arterial distensibility children and teenagers: normal evolution and the effect of childhood vasculitis. Arch Dis Child. 2002;87:348–351. doi: 10.1136/adc.87.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis. 2003;166:303–309. doi: 10.1016/s0021-9150(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 27.Joo SY, Cho KY, Cho SJ, Hong YM. Pulse wave velocity and ankle brachial index in hypertensive adolescents. J Korean Pediatr. 2006;49:769–776. [Google Scholar]