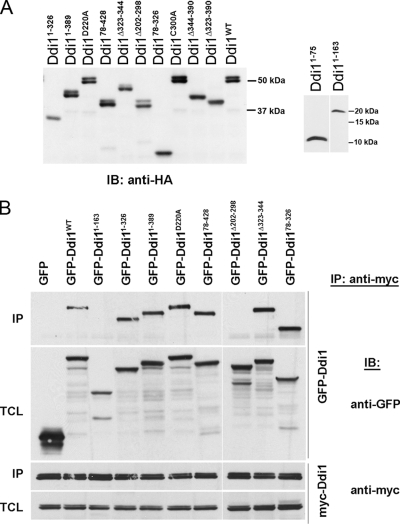
Figure 2.
Expression of Ddi1 mutants and mapping the regions of protein modification and homodimerization. (A) Verification of expression of the Ddi1 mutant proteins. Yeast lacking DDI1 (ddi1Δ) were transformed with multicopy plasmids overproducing wild-type HA-Ddi1 (Ddi1WT, pADH-HA-DDI1) or its HA-tagged mutants (Ddi11–75, Ddi11–163, Ddi11–326, Ddi11–389, Ddi178–428, Ddi178–326, Ddi1Δ202–298, Ddi1Δ323–344, Ddi1D220A, Ddi1Δ323–390, Ddi1Δ344–390, or Ddi1C300A), as indicated. Yeast were grown to the midlog phase, harvested, and lysed in 1% SDS/PBS buffer containing protease inhibitors. Aliquots of 20 μg protein/lane were resolved on 10% SDS-PAGE gels, whereas aliquots of 120 or 100 μg protein/lane were taken for Ddi11–75 and Ddi11–163, respectively, and resolved on 15% gels. Ddi1 detection was performed using anti-HA (1:1000) antibodies. Size markers (in kDa) are indicated on the right side of the blots. (B) Ddi1 undergoes homodimerization via the RVP domain. ddi1Δ cells were cotransformed with multicopy plasmids overproducing myc-Ddi1 (pADH-myc-DDI1) and one of the GFP-tagged Ddi1 proteins: (i.e., GFP-Ddi1WT or the GFP-Ddi1 mutants: GFP-Ddi11–163, GFP-Ddi11–326, GFP-Ddi11–389, GFP-Ddi178–428, GFP-Ddi178–326, GFP-Ddi1Δ202–298, GFP-Ddi1Δ323–344, GFP-Ddi1D220A or GFP alone), as indicated above the blots. Cells were grown to the midlog phase and subjected to immunoprecipitation via anti-myc antibodies. Detection of coprecipitated proteins in immunoblots (IB) was performed by anti-GFP (1:1000) and anti-myc (1:1000) antibodies, as indicated. Samples of both the immunoprecipitation (IP) and total cell lysates (TCL; 40 μg) are presented.