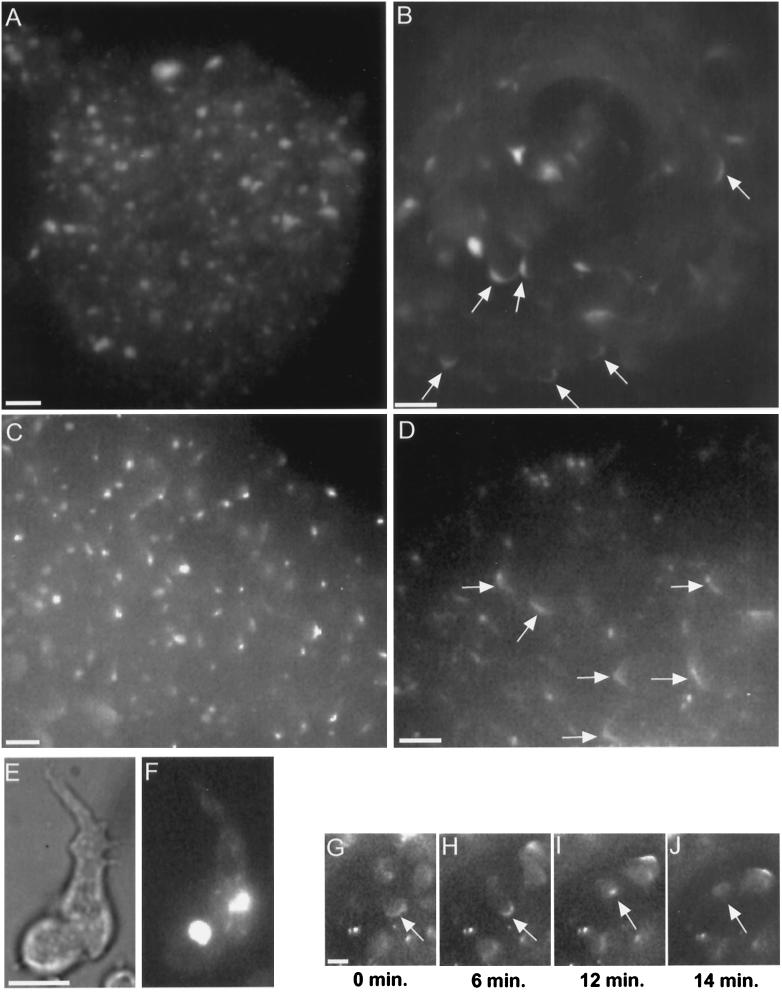
Figure 8.
Myosin II in RLC− cells. Starved cells with GFP-MHC reveal a bright myosin II aggregate (E, bright field; F, fluorescence), consistent with the distribution of myosin II previously observed by immunofluorescence in isolated RLC− cells (Chen et al., 1994). RLC− cells retain their GFP-MHC aggregate during aggregation and in the early mound (A). Cells in the mound shown in A were moving randomly, as is normal for early mounds. As rotation begins in RLC− mounds, the GFP-MHC aggregate disappears, and a cortical C pattern appears instead (B, arrows). The disappearance of the GFP-MHC aggregate is not an artifact of this GFP construct. Immunofluorescence staining reveals native myosin II aggregates in an early mound (C) and reveals Cs (arrows) and spot patterns in a later mound (D). Rotating RLC− mound cells with GFP-MHC exhibit a C-to-spot transition (G–J), including dissolution of the spot (J). The C-to-spot transition is much slower than normal (also see Figure 3A). Although the mound shown in B is composed entirely of RLC− cells, the generalized enhancement of GFP-MHC around cell perimeters is largely absent. Compare with Figure 1, C–F, which shows a mound also composed entirely of normal GFP-MHC cells, in which many individual cell outlines can be clearly discerned. Bars, 10 μm.