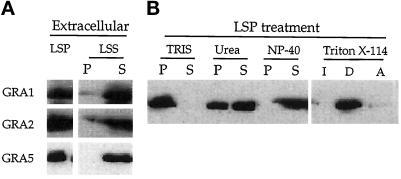
Figure 1.
GRA5 is found both in a soluble form and in hydrophobic aggregates within extracellular parasites. (A) Parasites were disrupted by F/T cycles and separated by low-speed centrifugation into cell ghosts (LSP) and a soluble fraction (LSS). The LSS was further fractionated by a high-speed spin into a membrane pellet (P) and a soluble fraction (S). Fractions were separated by SDS-PAGE, and the GRA1 (24 kDa), GRA2 (28 kDa), and GRA5 (21 kDa) proteins were revealed by Western blot using the TG17–43, TG17–179, and TG17–113 monoclonal antibodies, respectively. (B) The LSP obtained in A was resuspended in 50 mM Tris, pH 8.0, incubated with denaturing agents, and fractionated by high-speed centrifugation into a membrane pellet (P) and a soluble fraction (S). Fractions were analyzed by SDS-PAGE and Western blotting using the anti-GRA5 mAb TG17–113. GRA5 remaining in the LSP was partially solubilized by urea, completely released from the membrane pellet by NP-40, and partitioned into the detergent phase (D) after treatment with Triton X-114.