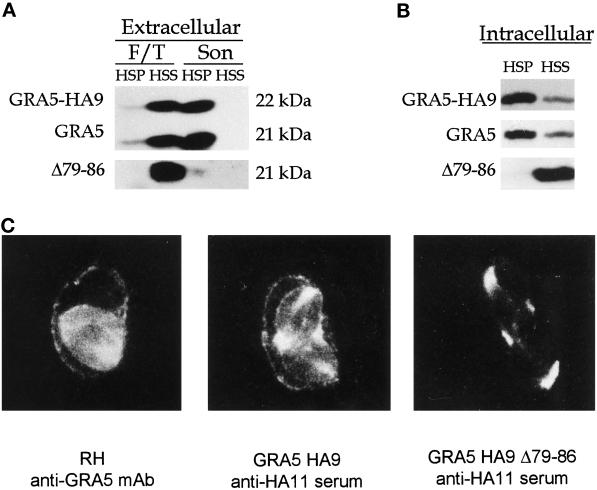
Figure 4.
Analysis of wild-type GRA5, GRA5–HA9, and the deletion mutant GRA5Δ79–86–HA9 by cell fractionation in both extracellular parasites (A) and the PV (B) and by immunofluorescence (C). (A) Extracellular parasites from the wild-type RH strain and from transgenic RH lines expressing either GRA5–HA9 or GRA5Δ79–86–HA9 were subjected to F/T treatment followed by low-speed centrifugation and fractionation of the supernatant into a 100,000 × g pellet (F/T, HSP) and supernatant (F/T, HSS). The wild-type GRA5 and GRA5–HA9 (both revealed by the anti-GRA5 mAb TG17–113) and GRA5Δ79–86–HA9 (revealed by anti-HA11 serum) were primarily detected in the soluble fraction. The LSP was further treated by sonication followed by fractionation of the LSS into a 100,000 × g pellet (Son, HSP) and supernatant (Son, HSS). The wild-type and epitope-tagged forms of the protein behaved as hydrophobic aggregates, being sedimented at 100,000 × g. In contrast, the deletion mutant (Δ79–86) was exclusively detected as a soluble protein. (B) PV proteins were fractionated as described in Figure 2. GRA5–HA9 and GRA5 were predominantly present as membrane-associated forms, whereas the Δ79–86 deletion mutant was released exclusively as a soluble form. The tagged proteins were both revealed by the anti-HA11 serum, whereas wild-type GRA5 was revealed by the TG17–113 mAb. (C) Immunolocalization of GRA5, GRA5–HA9, and GRA5Δ79–86–HA9 in infected cells is shown. Immunofluorescence was performed on cells infected with the wild-type RH strain (left), the full-length GRA5–HA9 clone (middle), and the deletion mutant GRA5Δ79–86–HA9 (right). The GRA5 and GRA5–HA9 proteins were both observed between intracellular parasites and associated with the vacuole membrane. In contrast, the deletion mutant was exclusively detected between parasites and was not found associated with the PVM or cytoplasmic extensions.