Figure 1.
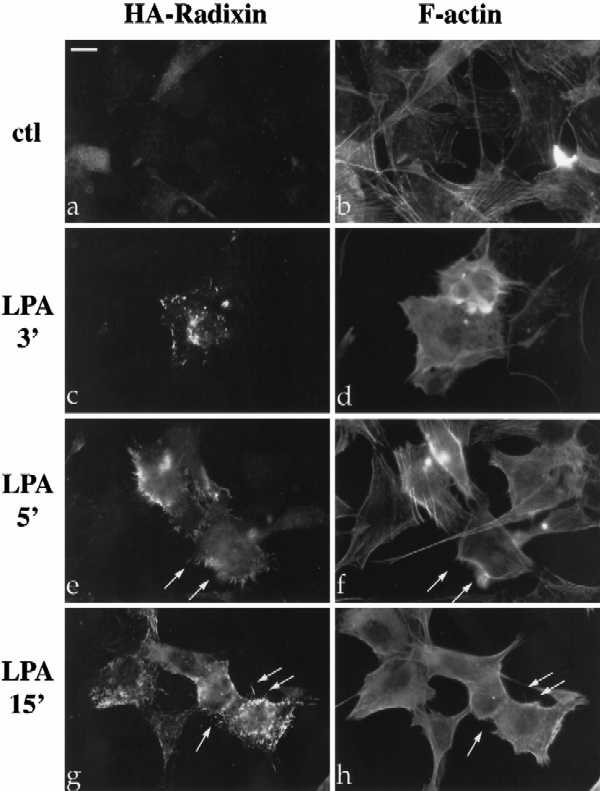
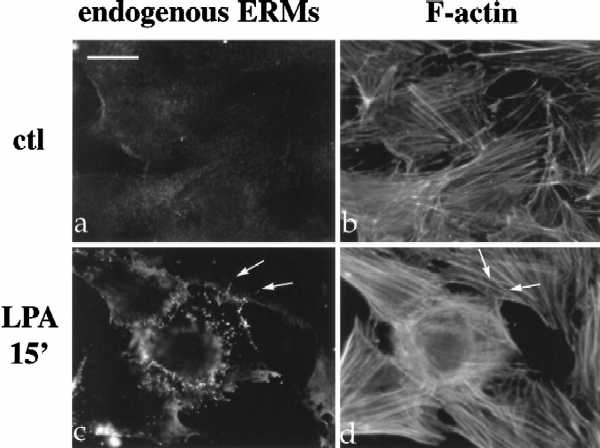
LPA-induced relocalization of radixin into apical membrane/actin protrusions in NIH3T3 cells. (A) Indirect immunofluorescence was performed on NIH3T3 cells stably expressing HA-epitope–tagged radixin. These cells were serum starved, and then treated with vehicle for 15 min (a and b), or with LPA (6 μM) for 3 min (c and d), 5 min (e and f), or 15 min (g and h). HA-radixin localization (using the 12CA5 mAb against the HA epitope) is shown in panels a, c, e, and g, and F-actin localization (using rhodamine phalloidin) is shown in b, d, f, and h. Starting 3–5 min after LPA treatment, radixin was relocalized into peripheral and apical membrane protrusions that also contain actin. These results were observed in three independent stable cell lines. Arrows denote colocalization of HA-radixin and actin in LPA-induced membrane protrusions. (B) NIH3T3 cells were serum starved and then treated with vehicle (a and b) or LPA for 15 min (c and d) and visualized for endogenous ERM proteins (a and c) using the CR22 polyclonal antibody or F-actin using rhodamine phalloidin (b and d). Arrows indicate the colocalization of ERM proteins and actin in membrane protrusions. Similar results were observed when a radixin-specific antibody was used as well. These results demonstrate that endogenous ERM proteins are similarly relocalized after LPA treatment as the exogenous epitope-tagged form of radixin. Bar, 10 μm.
