FIGURE 2.
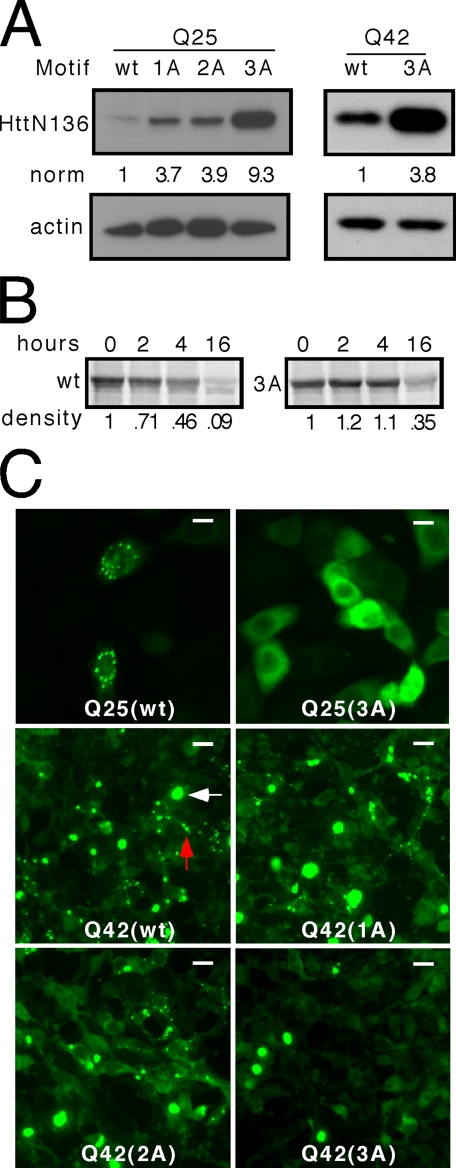
The FQKLL motif in Htt regulates steady state, degradation rate, and subcellular localization. A, sequential mutation of hydrophobic amino acids to alanine (1A, AQKLL; 2A, AQKAL; 3A, AQKAA) produces a progressive increase in the steady state level of HttN136YFP peptide with unexpanded (Q25) or expanded (Q42) polyglutamine tracts. B, pulse-chase analysis with quantification using densitometry indicates that unexpanded (Q25) HttN136YFP has a shorter half-life (∼4 h) than HttN136(3A)-YFP (∼13 h), implicating the role of the motif in protein degradation. All of the gels are representative of at least three experiments. norm, normalized band intensity/actin. C, HeLa cells were transiently transfected with unexpanded (Q25) or expanded (Q42) HttN136YFP containing wt or mutant (1A, 2A, 3A) motifs. For unexpanded HttN136YFP protein (Q25), the motif causes subcellular localization in discrete puncta, and the 3A mutation leads to diffuse distribution. For the expanded protein (Q42), classical cytoplasm inclusions form (white arrow). However, the motif causes additional subcellular localization in cytoplasm puncta (red arrow). When the motif is mutated by single (1A), double (2A), or triple (3A) alanine substitutions, localization to these puncta is progressively disrupted, whereas the inclusions remain. Scale bars, 10 μminthe top row and 20 μminthe lower rows.