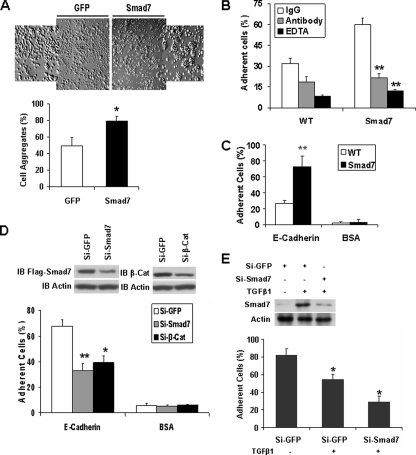
FIGURE 1.
Smad7 promotes E-cadherin-β-catenin-mediated cell-cell adhesion. A, single cell suspensions of T47D cells expressing GFP or FLAG-Smad7 were added to 24-well plates coated with 2% BSA; cell aggregates were photographed; and the percentage of aggregated cells compared with the total number of cells is shown. *, p < 0.05. B, calcein-labeled T47D cells with expression of FLAG-Smad7 or their parental cells were seeded on top of unlabeled monolayer of the corresponding cells in the presence or absence of EDTA (2 mm), the inhibitory anti-E-cadherin antibody, or the corresponding IgG. The cells were washed after a 30-min incubation, and the remaining fluorescence was calculated as the percentage of total fluorescence without washing. **, p < 0.01. C, calcein-stained wild-type (WT) or FLAG-Smad7 expressing T47D cells were seeded into E-cadherin- or BSA-coated wells of 96-well plate in triplicates. After a 30-min incubation, nonadherent cells were washed away with PBS, and the fluorescence of the remaining cells was quantified as a percentage of total fluorescence. D, FLAG-Smad7 expressing T47D cells were transfected with GFP siRNA, human Smad7 siRNA, or human β-catenin (β-Cat) siRNA, and 48 h later, Smad7 and β-catenin were detected by immunoblotting (IB). Analysis of cell adhesion to recombinant E-cadherin was performed as described in C. Knockdown of Smad7 or β-catenin reduced cell adhesion. E, NMuMG cells were transfected with GFP siRNA or mouse Smad7 siRNA, and 24 h later, the cells were treated with or without 10 ng/ml TGFβ1 overnight. Smad7 and Actin were then detected by Western blotting. Cell adhesion to recombinant E-cadherin was performed as described in C.